You would think that the rise in people with qualifications in cosmetic formulating would be closely mirrored by a rise in formulating competency and (one would hope) a rise in scientific literacy but that’s not been my experience.
I accept that my opening sentence may come across as a bit harsh. That it could indicate I’m a bitchy, stuck-up, better-than-you cow-bag that feels everyone should bow down to me, but that’s not what I’m about. My motivation comes from a mix of frustration, sadness and fear! Yes, believe it or not, I’m scared about where this is leading…
The Coronaverse created the perfect environment for self-evaluation and a change in working life! Some used that time to re-educate and follow a lifelong passion to launch a brand or business in the cosmetic space. To those, I say ‘good on you!’ This can be a very fun and creative place to work and play once you’ve got yourself orientated and educated!
In terms of education, greeting these newly primed beauty business bods down that rabbit hole were and still are a number of options and that, my friends, is where all hell breaks loose!
For those who are interested, this blog post has also been recorded as a Podcast and that’s available by following this link.
Hubris: exaggerated pride or self-confidence.
Education, tips and tricks – the good, the bad and the ugly!
The self-directed education options out there for beauty brand newbies are vast and exciting! Never before in my lifetime have there been so many choices, so much information shared and so much content available to consume. Choice is largely a good thing as it increases the chances that you, the education seeker will be able to find a provider or source of information that suits your needs, wants and budget. But choice can quickly turn sour when you realise much of what’s on offer is pretty rubbish!
After doing some of my own research (hahahahahahaha, that’s what they all say) I’ve become somewhat overwhelmed by the sheer number of people who’ve set themselves up as teachers, group leaders, facilitators, content creators, ‘experts’ or even whole schools on the back of what appear to be dubious, shallow and/or narrow foundations.
Here are some examples of what I’ve found:
- Season 1 ‘winners’ from the Coronaversity of life turned teacher
- That is, people who had quit their jobs and found some success with their own brands (or even just kitchen experiments) in the year before or right at the start of the pandemic, you know, at that time when our 2021-22 cohort were still wrestling that 6 pack of toilet roll from Karen in aisle 6. It is my opinion that some of these happy-go-lucky sharers and carers have possibly created so much soap or whipped body butter that some got into their eyes, preventing them from seeing that their experience and insights while somewhat helpful are extremely limited.
- Newly qualified graduates from reputable, accredited providers who go straight from zero to teaching / consultant hero!
- Now these are usually good courses so the information learned is likely to be relatively thorough, accurate and relevant. However, it’s never the best idea to go straight from education into teaching and /or consultancy without supervision and ongoing peer review. I’ve said many times that Cosmetic is an Applied rather than Theoretical Science so while one may have got a grasp on the theory from a course, it’s only after enough hours of practice that one can call themselves fully Qualified in a meaningful way. In terms of practice, for those wanting to teach or consult around cosmetic science/ formulating it really is essential to back up book learning with experience gained in an established industry setting over a sufficient length of time (I’d say at least 2 years to gain enough experience to get started).
- Newly qualified graduates from unaccredited providers going straight to teacher/ consultant or Facebook group advisor/ administrator roles.
- This is where most of my worry comes from and I’m sad to say this does indeed happen! I’m not sure what some of the unaccredited providers are teaching but some do seem to feeding the ego more than the intellect and that’s worrying. I’ve heard of cases where last years graduates teach this years students (what, why?). It’s worth noting that not all unaccredited providers/ courses are bad just as not all accredited providers are great, it’s just the variability is much greater when there’s no real rule book or oversight.
- Self-Taught brand owner turning their hand at contract manufacturing after doing a course on GMP or something similar.
- Everyone has to start somewhere and this seemingly wreckless jump into manufacturing oblivion could actually end up working out if the brand owner involved has enough money and insight to create a more experienced team around them. The red flag for me around this is again with the ‘self-taught’ part. Brand owners that do make a go of it (and many do) with or without any good, bad or ugly educational input typically succeed because they know what they know and are very good at managing and delivering that. Contract manufacturing, white label, formulating and tweaking your products to suit others may sound like a no-brainer after doing all that hard work yourself (and winning at it) but in reality it’s a ball-ache that brings millions of new issues and struggles. I’ve heard from a few people in this camp who have ended up over their heads in issues after diving into this playground on their own.
- Smart Entrepreneurs who turn their special interest in cosmetics into a whole educational business model.
- It was 2003 when I finally graduated with my Diploma in Cosmetic Formulating from the London School of Fashion. In the 19 years that have followed everything has changed. I completed that course while working in my first cosmetic industry job. I worked out of orange covered work-books, attended in-person summer school at Boots the chemist in the UK and spent evenings at Society of Cosmetic Scientists lectures all over the UK. I know I had a computer at home for some of the coursework but also know that other work was hand-written and submitted via snail mail. These days courses are often conducted online with work-at-your-own-pace delivery schedule. Content is multi-media and three-dimensional. Supplementary information and examples are being freely provided on You Tube and in Social Media groups or via Teams. This has the potential to be great! Whether it is great or not still rests with the quality of the course content and teaching, things that can’t be short-cut, jazzed up or over-looked.
I can’t deny being angry about this situation. For me, this feeling stems from the fact that this, cosmetic chemistry, is all I’ve ever been good at and valued for in the business world and now it feels like it’s being unvalued, trashed even. Don’t worry, I’ll go work on that with my therapist…
In the spirit of playing the issue not the human, those who identify as being in any of the above scenarios are neither to be shamed nor, entirely blamed for the role they are playing in lowering the scientific integrity bar in this industry. For the most part, these people have good reason to feel they can go ahead and establish or diversify their businesses. Many are motivated by the desire to empower others; some want to give back to the industry that welcomed them in and gave them a diploma; others identified a niche or opportunity and just ran with it! That is all worth celebrating, that’s what businesses do!
The bottom line is that most of these people didn’t just rock up here on a whim. First, they invested; put in the hours; went to ‘school’ and got themselves an education.
With that in mind I need to draw my thoughts, this blog post, to a conclusion.
I still don’t really want to admit it but I feel I must.
It’s not the bad schools, the naive, inexperienced but enthusiastic teachers and graduates, the business-savvy-but-science-light entrepreneurs or crafter-turned-pro that are at fault here. No, this is my failure, a failure of me as a Cosmetic Industry Trained Chemist. It’s a failure of my peers and my industry.
Collectively ‘we’ have failed to preserve and promote the foundational role academic integrity and scientific method play in what we do. We’ve failed to explain how our adherence to, respect for and application of these fundamentals create opportunities for better, safer, greener, cleaner and more exciting products. We failed to win hearts and minds.
We created this monster and now this monster is suffocating us.
Carbomer Chemistry & High Speed Mixing / Homogenising.
The other week I was made aware of a new ‘rule’ that’s making its way around the virtual hallways of cosmetic school (which one, who said what and why doesn’t matter), the rule being that you should not homogenise Carbomers. Now I’m not sure of the wider context of this truth or how it was originally meant to be taken but it was clear that in this case the statement had been taken as literal truth and that was causing a problem.
This Blog Post is also available as a Podcast. If you would like to listen to that, please follow this link.
For those to whom the word ‘Carbomer’ is new, Carbomers are not a group of political insurgents intent on causing collateral damage, they are a group of polymeric ingredients used for thickening and suspending things in a formula. Many grades of Carbomer exist and nearly all of them create crystal clear thickening gels which impart an elegant non-tacky skin feel, have high suspending power/ Yield strength and help stabilise emulsions. These features make them very popular in the world of cosmetic science and as such, it’s very important for a formulator to know how to handle and formulate with them.
In terms of what a polymer is, polymers can be natural or synthetic and are made by creating chemically bonded chains of smaller units called ‘monomers’ together. Natural polymers include starch, a polymer made from glucose monomer units and hyaluronic acid, a polymer with monomers of D-glucuronic acid and N-acetyl-D-glucosamine – two disaccharide sugars.
Carbomer polymers are made from Acrylic Acid monomer, a simple carboxylic acid created through the oxidation of propylene. Propylene is a bi-product of gas production and so in the past and to this day, Carbomers are classed as ‘synthetic’ ingredients – ingredients derived from fossil fuels.
Carbomer History in a Nutshell.
Carbomer polymers were first commercialised in 1958 by the B.F Goodrich company with the trade name Carbopol. Today Cosmetic Chemists, especially those my age and older, may use the terms Carbomer and Carbopol interchangeably to refer to this chemistry. This is mainly because for a long time the Goodrich polymer range was either the only or the superior option on the market. Over the years the business changed hands, other players came into the market and options opened up. However the blur between the commercial and technical name for this chemical family persists and that’s relevant because in some cases, students of cosmetic science who are learning outside a factory/ commercially scaled-up laboratory setting are showing signs that they are missing the nuance in this little ingredient corner. So before we go on, I want you to appreciate that carbomers/ carbopols are a chemical family rather than just one thing; are a type of chemistry that’s now made by a range of manufacturers in a range of distinct grades; that different grades of carbomer (I’ll use that term from now on) have different features and benefits and that it makes no sense to view this chemistry with a one-size fits-all lens.
Defining ‘Homogenise’.
Thinking back to the rule we are exploring: ‘Never homogenise Carbomers’ it’s time to see what we mean by homogenise.
Just as we saw the words Carbomer/ Carbopol can be squished and squooshed up in our heads and taken to be the same, singular thing (reduced & homogenised), so can the term homogenised/ homogenisation.
While I may be pedantic, detailed and precise in my teaching practice, when I’m just being me I can be quite careless with my words and that may be what’s happened here. I’m potentially being more generous in entertaining that idea than my gut wants me to be but hey, life is better if we all think the best so here goes…
Homogenisation / homogenising / Homogenise CAN be terms that are thrown around in a cosmetic lab and taken to mean any of the following:
a) Any method of mixing that achieves a homogenous outcome.
b) Any method of mixing that achieves an optimally-dispersed outcome.
b) The specific process of using a tool that’s called a homogeniser.
c) Slang/ shorthand for mixing that happens at a higher intensity than is required to just blend ingredients together, maybe to achieve outcome a or b OR to aerate the product or just ensure it’s all blended together.
Confusing isn’t it!
Back to the original reason, I’m doing this investigation – the scenario the person speaking to me had found themselves in.
The person I spoke to was struggling to get their powdered carbomer to mix into their water phase without ‘fish eyes’ (blobs of dry polymer with partially hydrated polymer jelly around them) forming. They had a separate homogeniser machine to hand but had not tried it due to what they had been told and instead were using their propeller mixer for this stage.
The formation of fish eyes is a common problem with Carbomers, especially the basic, simple types – later versions are often manufactured to be ‘self-wetting’ or ‘quick-to-disperse’ making this wetting out stage much easier but this grade isn’t so these fish eyes were a problem!
What I do when working with Carbomers is have my water phase warmed up and moving first, I then sprinkle the Carbomer into the vortex made by my propeller mixer as if I’m dusting a cake with icing sugar. I usually have my mixer on a medium speed (maybe 1500-2000 rpm ish) although I gauge the RPM more by sight than measurement as it depends on the batch size, the vessel I’m using, how much Carbomer I’m trying to hydrate, whether there’s any other chemical in the water and the water temperature. It all matters (detail you see). Anyway…
Once all of my powdered Carbomer is in the water and mixed around somewhat I have a look at it. If it looks like it is going in nicely and if I have time to let it do its thing I’ll leave it here and give it time enough to fully wet without moving it to my homogeniser, this could take anything from 5 to 20 minutes for a laboratory batch. If I’m in a hurry or just can’t be bothered I’ll pop this under my homogeniser and give it a whirl to speed up the wetting process.
OH NO, DID SHE SAY SHE HOMOGENISES CARBOMER?
Yes I did…
YOU NAUGHTY GIRL, WE WERE TOLD NOT TO DO THAT!
Yes but I think that rule is utter bollocks.
Carbomer Through The Stages.
Many, but not all grades of Carbomer go through a step-by-step metamorphosis process before they become the rheology modifiers of your dreams. Each step of this process is different and should be treated as such.
My question now is this: Is whatever problem Carbomer’s have with homogenising (whatever that means) equal across every stage or more important for some stages? Let’s dig further…
- The Dry Powder Stage.
- Many (but not all) carbomers come supplied as powders. These powders may be described as self-wetting, self-neutralising or they may be just regular powders. In any case your Carbomer, if it is a powder, is going to be easier for you to handle if it is in a free-flowing state. As with any powdered ingredient, Carbomer powders can become lumpy and/or compressed (solid). Look at your powder before adding it to your formula and if it is either of those things and ESPECIALLY if it is not a self-wetting type, pre-work your powder by breaking up the lumps into free flowing powder/ particles again.
- Getting it WET/ Wetting out your powder.
- Adding your Carbomer powder to the water phase is not the only way to get Carbomer into a formula but it’s the way I’m talking about here because this is where at least some of the homogenisation need/ confusion comes from. There is a difference between getting your powder wet and fully wetting out your powder, we are aiming for the latter. Wetting out a powder means that each individual particle of Carbomer powder has been touched and penetrated (ooooohhh eerrr Mrs) by the water, just getting it a bit wet is what gives you fish eyes and problems. If you fail to optimise this stage, you will fail to achieve the maximum viscosity and stability for the amount of Carbomer you’ve added, regardless of what you do next. This is a very important step!
- Neutralising the powder
- Not all Carbomer chemistry need neutralising by the formulator/ manufacturer but many do. For those grades that do need neutralising, as supplied, the Carbomer is in its acid form and we neutralise it with a relevant alkali to complete its metamorphasis and give us our complete gel. It’s common in manufacturing for neutralising to be one of the very last steps before taking the product out of the mixer and into storage. It’s relatively uncommon (although not entirely unheard of) for the Carbomer to be neutralised early in the piece. Once neutralised, the viscosity of the finished product typically increases and any mixing you want to do after this requires more energy input as there’s more resistance. Is it possible that the ‘don’t homogenise Carbomer’ rule is talking about step 3 only or mainly? I feel it may be and even then it may not always be a black or white issue.
Important Step Along Your ‘Carbomer’ Journey.
For your Carbomer to reach its maximum viscosity it needs to be fully wetted. For the Carbomer to be fully wetted, it needs to be able to experience the water phase (whenever it is introduced to it) to its full potential. This is more likely when you’ve maximised your Carbomer’s surface area by breaking down any compacted, lumpy powder and you’ve preventing any fish eyes from forming through adequate and optimal mixing. You’ll generally find water that is warmer hydrates Carbomer powder faster than water that is colder and faster mixing avoids fish-eyes and fully wets the powder more efficiently than no or slow mixing.
And this is why I feel leaving students with the idea that homogenising carbomer containing formulations is wrong is very unhelpful.
Nuance, detail and context matters and that’s why I both LOVE cosmetic science and HATE it when people teach it with broad, shallow brush strokes. It may be technically right, at least on one level to use caution when using a homogenisation step with Carbomer in some scenarios but impractical and potentially problem CAUSING if taken too broadly and/or literally.
Going back to what I said about homogenisers and how the term ‘homogenise’ is used by cosmetic chemists in a practical, factory setting.
I would describe how I ‘wet’ my Carbomer powder as ‘homogenising’ on my formula write-up meaning I’d typically use my homogeniser at a relatively low speed (3000-5000 RPM) to help reduce the potential for fish-eyes, speed up wetting and minimise the time taken on the plant (time is money in cosmetic scale-up). I would then typically note the end point as the point at which the powder appears fully wetted (partially swelled), with no visible fish-eyes. I may then suggest returning to the propeller mixer to complete the wetting if needs be, depending on how much Carbomer was in the formula, how much free-water was there for it to ‘wet’ into (remembering that not all wet stuff is water, glycerin and other humectants can make water unavailable for the swelling), and what temperature constraints I had in the water phase. However, it may be more accurate of me to swap out the word ‘homogenise’ for ‘disperse’. Now this would be pedantic as in my laboratory setting the mixer I call my homogeniser is actually sold as a dispersing tool (just to confuse everyone) but the way it mixes is the type of force that the manufacturers of Carbomer avoid caution with so with that in mind I’m going to be careful not to disperse/ homogenise the crap out of my Carbomer at any stage. As with all tools one can use them gently or otherwise and the outcome you achieve will vary accordingly.
Is There Any Evidence To Suggest Homogenising Carbomer Is Bad?
Yes there is.
If we set aside what I’ve said about the semantics of language cosmetic chemists use around the term ‘homogenising’, using a mixer capable of creating high levels of friction and/ or a mixer that has a very fine chopping and/or grinding motion can damage polymeric thickeners such as Carbomers. Indeed, the manufacturers of Carbomer do caution against all-out homogenisation. This advice is typically directed at the neutralised rather than pre-wetted Carbomer but it isn’t always made crystal clear and maybe that, plus a lack of experience of how factories operate, has contributed to the level of confusion we now find ourselves in. Well that and the fact, pre-neutralised Carbomer and/or other acrylate thickeners may be more suceptible to homogenisation viscosity loss than the old-fashioned and more common types.
So What Does Homogenising Do To Carbomers?
As the shear force and/or temperature the polymer is exposed to increases, the chances of damaging the polymer chains rises. Based on experimental data I’ve seen to date, the exact ‘break point’ varies, depending on whether the polymer has been neutralised or not, how concentrated it is, what grade of polymer it is and the exact type of mixer it is being exposed to. This damage manifests as a non-recoverable loss of viscosity – so the end gel or product is thinner than it otherwise could be.
In a laboratory setting, it is possible to test all manner of different variables to whatever limits you can achieve. Not every scenario that can be created in a lab is likely or even possible experienced in real-life. Most factory equipment and time constraints mean that maximum speeds and mixing durations are hardly even reached in practice. What is relevant and what needs to be managed is what’s likely and then what’s possible.
Who is Right? What Rule Do We Adopt?
I would answer this by urging you to think about the Carbomer/ Homogeniser in the context of your specific formula and go from there.
If you are making an emulsion and using Carbomer as your water-phase thickener/ rheology modifier it probably makes sense to use a homogeniser to ensure fast and full wetting of the Carbomer prior to neutralisation. In this case you are saying Homogenisation but meaning ‘dispersing’ – tool will likely be the same but outcome is visually different.
Homogenisation isn’t an all-or-nothing mixing situation so in an emulsion setting you could experiment with different speeds and mixing times to get the maximum benefit with minimum risk (remembering risk is not just damage to the Carbomer but (more likely) failure to fully wet out the Carbomer).
In the case of creating an emulsion, it’s all very well everyone talking about the way high shear homogenisation results in a permanent reduction (up to 50%) in Carbomer viscosity but if you don’t consider the fact that such high shear is unlikely in most cosmetic manufacturing settings, a shear rate that is too low is almost guaranteed to leave at least some of the Carbomer powder either un-wetted of sub-optimally wetted and that will cause more than just viscosity loss. It is also worth mentioning that most Carbomer grades we use in cosmetic formulating achieve their maximum viscosity as a function of pH. Most cosmetic products are set to a pH of the skin rather than the maximum pH for the Carbomer so you are unlikely to be needing the maximum viscosity possible from your Carbomer anyway. So risking a little Carbomer breakage then rectifying it (if necessary) with a slight pH adjustment is more sensible than the alternative which is not recoverable once the wetting stage is past. Finally keep in mind that you can homogenise a cosmetic emulsion BEFORE neutralising your Carbomer, in fact, this is most likely the norm.
In the case that you are making a gel, especially one that stretches the limits of the Carbomers salt, solvent, surfactant or free-water tolerance, it may well be more detrimental to homogenise the neutralised gel, especially at high speed. However, in the vast majority of formula scenarios this isn’t going to be necessary or aesthetically pleasing. Homogenising a fully neutralised gel may trap air causing a visual bubbling effect, change in your specific gravity and higher potential for negative micro and oxidation results. While all of these things (once known) could be mitigated against and some level of homomgenisation achieved, it is less likely in general that one would homogenise (especially at high speed) this type of formula.
The Bottom Line – Summary.
Anyone who is involved in sharing, teaching or passing on information about Cosmetic Science (including me – and I know I’m not above making my own mistakes) should keep in mind the APPLIED nature of this science and the complexities and nuance involved in each formula, grade of ingredient and manufacturing setting. I typically caution against reducing things to ‘always’ and ‘never’ statements, especially when they are left unqualified as this can lead to misunderstandings and confusion.
Sure, the world of nuance, multiple meanings and vagueness is also confusing but at least it gives you more to question and test and THAT’S what it is all about.
For those that skip straight to the last line the answer is as follows:
Homogeniser style mixing can damage Carbomers in a way that permanently reduces their capacity to reach maximum viscosity. Whether using a homogeniser does this or not depends on many things including the stage in which you homogenise (wetting, pre neutralisation or post neutralisation), the style of homogeniser you use (machine), the time/ speed settings employed and the overall character of the formula. In a practical setting, there is plenty of evidence and many scenarios where the extra speed and power a homogenising step brings improves overall formula outcome and reduces the chances of stability, aesthetic and microbial problems. Therefore it is unhelpful to hold the belief that homogenisers should never be used with Carbomers.
Useful Resources
I really like this article on Carbomers written by Mike J.Fevola of Johnson and Johnson even though he also states that you shouldn’t homogenise Carbomers. He’s definitely with the mainstream in using that throw-away line and I won’t judge him too harshly for it as he’s not all wrong and it’s not the main take-home from the article.
This report from 2014 by Lyndel Speedy from Monash laboratory in partnership with Ensign Laboratories (hello chaps) is also a good read and one that seems to confirm the opposite of what I’m saying (i.e: they are showing why you shouldn’t homogenise Carbomers). However, this primarily investigates Carbomers after neutralisation so it is very useful for people using Carbomers but doesn’t really talk about the wetting step in any detail. When it does I don’t actually think their method was that fair as the homogenised samples got way less time to wet -and wetting still takes some time. Anyway, it’s still good in terms of getting you thinking.
This paper on mdpi is quite a good overview of Carbomer rheology. It’s very detailed and good for those wanting to understand the chemistry and rheological flow to a greater degree.
Here is a link to Lubrizol who own the Carbomer range now. This gives you an entry point into rheology, shear and the different polymers in their range. They have a lot of resources that are helpful.
I also found this useful from Silverson. Silverson are manufacturers of the industry standard ‘homogeniser’ so it was good to hear from them with regards to mixing, wetting and neutralising Carbomer. It was through reading this that I realised that I had to talk about equipment as well as the action of homogenisation.
One last paper I’ll share is this one which goes into more detail about the rheology of carbomers. One thing that interests me still is this idea of shear ‘breaking’ the polymers. The rheology of Carbomers as a family is quite complex and is also linked to the concentration of the Carbomer in solution. I am still not sure about size and shape of the safe zone for using high shear mixing with Carbomer in general and have found it hard to find a paper that really explores viscosity changes over time and different pH after different shear. It’s often you get one or two variables but not all three.
The world is made up of in-groups and out-groups and generally, that sucks! Sometimes, the in-groups are small and transient, like that group of girlfriends from school, the ones that decided collectively that they don’t like you so you can’t sit with them at recess today because you like the ‘wrong’ music or have the ‘wrong’ shoes. But sometimes the in-groups are so big and permanent, so all-encompassing that we just accept them or fail to recognise them for what they really are, or the harm that they can do.
The global cosmetic industry is an in-group of sorts and that’s what my podcast (follow this link for 40 minutes of reflection on that topic) is about. But for those who either can’t stand the sound of my voice, can’t be bothered to listen to a podcast or are only here for the cheap thrills and summary keep reading. Actually, everybody keep reading a bit because I free-styled the podcast (didn’t make notes) so it’s more flowy and natural than when I read it but it also contains some references that I think work better when you can see what I’m thinking of in my minds eye. So do yourself a favour and suck it all up through a great big noodle-like straw. Yum…
The White Gaze Over The Global Cosmetic Industry

The main points covered in my podcast are here. I’ve not mirrored the podcast though so you’ll pick up different things there to here although they do cross-over of course.
- The global cosmetic industry has a style, aesthetic, norm and way of operating that stems from it being a white, western construct. While the industry has become more diverse and open, like any other system that was established around a narrow, victor-style worldview, the industry still has many built-in blind spots.
- The dominant narrative around the global cosmetic industry is still prioritising the wants, needs, aspirations, desires and norms of a white, western target market. The way this manifests includes how we talk about cosmetic and personal care products, the types of products we make, the beauty regimens we normalise and the outcomes sought. It also includes the way brands present themselves onto the market.
- Scanning the global cosmetic industry shows just how homogenous the market is around the world. There is a definite aesthetic that dominates how a cosmetic product should look and at the moment that’s in line with global interior design trends (which are also from the white western perspective). The ‘norm’ for boutique/ niche brands, lifestyle brands and clinic ranges is minimaist and neutral. Dominant colour schemes for packaging are typically black, white or neutrals. Multi-nationals take advantage of their ability to create their own brand identity and colour scheme and have largely stuck with visual signs of opulance, luxury and cleanliness with their gold, silver, pearly white, blues or reds. Both multi-national and smaller brands adopt a globalised approach to their branding as a whole which I will define next.
- The globalised approach to branding is what I tend to call a ‘blanding’. This is me being judgemental but as I see it, it’s a safe (well, from the Western gaze) construct divorced of deeper meaning. A global cosmetic industry would stuggle to penetrate if we were all wedded to our culture or more nuanced societal identities, our sensual selves, our meat and bone beings -as for intersectionality, don’t even go there! Instead, the global cosmetic industry first divided us into skin types (dry, oily, normal etc) before selling us solutions to balance our deficits or problems. Now it’s gone a bit further and is appeal to us on a deeper level using our values. Only these values are not so much personal and how we feel as they are political and what we think – think sustainable, vegan, ethical, vegan, free from/ safe, green, natural etc. While these two ways of organising people so as to sell them something they want are not bad, they have created a norm that is a cultural wasteland. This deculturisation or homogenisation of people is so ingrained as our norm that brands that join culture with product stand out a mile and look different.
- People that sit outside of this white, western identity can and do create brands that are successful in this space. People that do identify as white and western can and do create brands that sit outside of it. This isn’t necessarily about how you personally identify or what you CAN or SHOULD do, this is about understanding the dominant game as it is and intentionally choosing how you interact with it so that you have the power.
- When thinking of your potential or existing brand your whole self can show up in a number of ways, each of which is on a spectrum that you can explore to the max or just dip in a little:
- Packaging choice – materials, finish cut style, size etc. It may be culturally or personally significant for you to use clay pots as packaging and these might also tap into the mainstream trend of being zero-waste, recyclable, reusable. Or you might want to use ocean plastic in your bottles to make a subtle statement around your cultural or familial relationship to the sea. You might choose a bottle or container shape that has ancestrial meaning or choose to wrap your products in a culturally significant way rather than use a standard label.
- Colour scheme on packaging – colours mean different things to different people. Don’t be afraid of shunning white (a symbol of mourning in some cultures) and going with red, yellow or green as your base colour. Alternatively use splashes of colour or a colour pallet that’s meaningful in a way that’s subtle but that brings a layer to your narrative.
- Graphic Design/ Visual Stories – A culturally relevant logo or brand icon won’t necessarily make sense on a product that’s otherwise subscribing to the white western narrative but when paired with other layers of marketing, it can be a very obvious and attractive way to reach your people. These graphics can extend beyond a logo to include patterns, materials, art practices and styles that are meaningful to you and your target group.
- Ingredient choice – many people like to mention that their product features interesting ingredients but just mentioning you use Brazilian botanicals or Australian Native Bush Flowers, Ayurvedic medicine or Chinese herbs without adding a deeper narrative reduces their value. They become commodities that have been ‘bought’ or are being ‘used’ to serve the dominant narrative.
- Product Range Choice – The dominant skin and haircare regimen promoted by the global cosmetic industry may not be the best way forward for you and your brand. Don’t be afraid to assert your individuality and explore your cultural norms by switching things up in a way that best suits you and your target market.
- Language – English is the dominant language of the global cosmetic industry but this may not be the language you and your target audience are most comfortable with. I’ve seen brands use their language in creative ways for branding, in product names and to describe routines and practices. Strategic use of non-English language can be intriguing for the white western audience while being deeply comforting and a powerful way of connecting to your core target market. Then there’s what you say and how you say it. You can weave your individuality and group identity into this in ways that are subtle for the mainstream but will resonate with your core group.
- Formulations – last but not least is the stuff you put into the packages. Your formulations can and should be made to target the needs, wants and aspirations of your target audience while fitting their lifestyle and lived reality. This could manifest in terms of the weight and texture of the cream-based emulsions you make, the intensity and tone of the colours on offer or the way the product reaches its desired outcomes. This very subtle way to mark your territory is perhapse the most powerful and is what will keep customers coming back. Great formulations are what has helped Rhianna and her Fenty Beauty retain its audience and brand following now that the initial excitement over the range of shades has died down!
Summary.
The cosmetic industry is changing but it is changing slowly. Sure there are now more people of colour and people who don’t identify as white and western, developing their own brands and running their own businesses but this on its own isn’t enough. It’s not unusual for people outside of the mainstream to enter the cosmetic industry with a brand that conforms to the unwritten style guide of the global cosmetic industry. That’s fine when it’s done with intention but more often than not the choice is made subonsciously out of a lack of alternative options, either perceived or real. Don’t become ‘that’, don’t bland yourself down or abandon your perspective, narrative or insight. Instead, let ‘that’ become you – let the global industry shimmy along and make space for all that you wish to share in the way that you wish to share it. I’m passionate about broadening the narrative in all directions and yes, that includes my own white, western one as I’m not even so sure we got that one right.
I hope that makes sense and if you need any help in working out how to make this approach work for you and yours, drop me a line.
Amanda x
PS: Once again, the Podcast link is here: Podcast ‘Room To Play, The Global Cosmetic Industry and You, The Outsider’.
Somatosensory means ‘of the body’. The term embraces the three ways in which we experience the physical world. Proprioception focuses on the movement of bone and muscle. Exteroception centres around interpreting touch, Interoception focuses on the status of our organs (heart rate, breathing, digestion etc).
A podcast of this article is available by following this link to Spotify.
The way we do science in the western world often starts by deconstructing a thing, taking it apart and examining each part in isolation from the others. This method has its uses, it lessens the complexity, makes things easier to learn, cuts down background noise and allows for simple, more convenient experiments. But humans aren’t simple, we are far more than the sum of our biological parts (or at least we feel we are). We are often so difficult to predict and know that most of us confess to not even understanding ourselves at least some of the time. But this is about cosmetic science, not psychology so it makes sense to start our navigation of the somatosensory world through our sense of touch.
Before I go on I’ll explain how and why I became fascinated by this topic.
Often life feel like an assault on my senses. I struggle to navigate space and am very clumsy; have skin that’s highly sensitive to light fluttery touch but that enjoys and even seeks out deep more constant pressure. I love loud music but struggle with multi-layer man-made background noise of any frequency. I struggle to read signs that I’m getting too hot or cold and as a consequence often end up with heat stroke or frosted fingers and toes.
Last year, after seeking an explanation for all of this I finally got my answer. I am autistic and these struggles stem from a somatosensory system that ‘works’ outside of what society classes as ‘normal’. So while my body responds in a biologically normal way to sensory signals, my mind doesn’t interpret those signals along with societal norms. Sometimes I am MORE affected by these sensory inputs and sometimes I’m less. Another way this shows up is as a delay or out-of-synch processing which can mean I miss ideal time to respond to a trigger (in conversation, going to the bathroom, going to sleep, getting out of the sun etc).
Learning this about my self started my mind whirring. The skin’s role in our somatosensory system is huge, I’m a cosmetic chemist so the skin is a huge part of my work. This was something I just had to get to know better and that’s why we are here!
The somatosensory system is triggered by our five externally focused senses of taste, touch, sight, sound, smell but goes way beyond the mere tangible and directly measurable. It is through this system that we can describe in detail not just how something feels but how it makes us feel.
This is the system that helps us explain how we decide something feels itchy, hot or heavy to us when other people are experiencing it as unremarkable. I feel that if we, as cosmetic industry professionals can work with this system in an integrated rather than reductive way, a way that resonates with our whole consciousness, we will not only make functionally better products but we will make products in a better, more efficient way. That we will be able to pinpoint accurately and concisely what will hit the spot, rather than the more common (but more haphazard) formulating method that sees us throwing everything at it and in it in the hope that some of it sticks (in a non-sticky, aesthetically elegant way of course)!
The Somatic Sense of Touch.
For the purpose of this exploration, I’m going to focus on exteroception, the part of our somatosensory system that interprets touch. I’m not denying that the formulations I make and the products they become must appeal to all the senses to be successful but our sense of smell and appreciation for a slick visual aesthetic has held the spotlight for too long already. It’s time for this more intimate and nuanced product: client interaction to come first!
In this scenario, the ‘world’ to be interpreted is the cosmetic product, the feedback, that which originates from the skin.
It’s important to note this is not just about our choice of chemicals
Whether or not the chemicals we formulate with are classified as nice or nasty, natural or synthetic, potentially irritating or gentle is only part of this and, I dare say, the part we’ve focused on almost exclusively up to now. This is about the product as one – the way it presents is dispensed and applied. The way it dries down, slides around, sticks or rinses off. This is about our wider relationship with it – how often the product is used, what happens afterwards and how long any changes persist. It’s important not to get stuck in a narrow focus that’s off to the side of this main sensory highway.
Exploring our largest organ.
The relationship between the skin and the cosmetic products we apply to it is dynamic, complex and changeable, a true ‘applied’ relationship.
We expect this relationship to have a biological component, that the products – the chemicals – we use will affect the skin in some way and cause a dose-linked response. But the impact a product has on our senses goes much further and deeper than that.
The skin, in a cosmetic sense is predominantly the epidermis, the stuff we see, the layer we wear. Occasionally cosmetics triggers a cascade of changes that reach further, past the dermal/ epidermal junction and into the dermis.
There are chemicals that we can track as they make their way from the epidermis all the way through the skin and into the bloodstream. Some wanted and helpful, others less so. The fact that some cosmetic products contain chemicals that can gain access like that sometimes scares us, makes us re-think our choices and habits. Makes us question why we do what we do. But these traceable foreign chemicals are only part of the story as is any investigation into the correlation between what we think about them and the biological impact they have but that’s something we can discuss another time…
Cosmetic products can and do trigger an entirely different set of chemical cascades in our bodies and that’s what we are going to focus on here. This is the story of the senses and what happens when they are awoken and triggered.
How does it feel?

Our skin contains a range of different sensory receptors which we refer to collectively as nerves.
Our nerves respond to information they receive from the outside world, sending chemical signals through our nervous system and to the brain. Here the brain translates these signals into feelings. Our nerves exist in different shapes and sizes and at different positions in the skin and around the body. We have nerve cells that sense texture, those that sense weight (pressure), position and threat (danger or pain). The podcast of this article gives some examples of how these four sensory boxes may be used by the cosmetic chemist.
The way we describe these feelings to ourselves and others, whether we enjoy or fear them, feel them a lot or a little, interpret them as threatening or helpful differs from person to person.
While there is good correlation across the human population around how sensory signals are interpreted at a basic, instinctual level, that layer of interpretation is typically left for medicine. Cosmetic Science focuses on the more nuanced and subtle layer of interpretation the one that is shaped by our life experiences and individual nuances.
Cultural, societal, historical and individual biological factors play a huge part in shaping our response to nerve stimuli. These factors all make up what psychologists call our ‘window of tolerance’ and this in turn is made wider or narrower by our expectations and sense of control.
Our Window-Of-Tolerance
The window in which we are most comfortable and can function at our best. Most everyday cosmetic products are formulated to sit within our window of tolerance. Cosmetic chemists spend a long time focusing on how a product feels and creating textures that disappear into or feel like they have become the skin. We don’t usually want to spend the day focused on feeling like we have something foreign wiped over us.
There are times when we want to raise our level of stimulation and create a sensation on the skin.
It is not unusual for clients in a medispa or semi-therapeutic setting to want to ‘feel’ the product work.
Think of AHA treatment products, Exfoliating scrubs, clay treatments that dry on the skin, film-forming masks and treatments, products that heat up on use, products that cool us down, toners that smart and perfumes.
We may want our cosmetic products to help calm us down and soothe us. Either to bring an elevated nervous system back to calm such as in a cooling and soothing after-sun product or post-waxing gel, a spot treatment cream or rich hand cream to smooth over sensitive cracks and dry spots and help us go about our day.
At other times we may want our product or to help us achieve or remain relaxed such as in a calming massage treatment, epsom salt bath or aromatherapy treatment.
Target Market Specific Window-Of-Tolerance
Cosmetic brands are typically designed around target markets with similar skin types or concerns. Brands and/or product ranges may also target specific age groups, genders or life stages.
It is not unreasonable to assume these target markets will share similarities in their somatosensory window-of-tolerance.
Understanding this at a deeper level helps brand owners and formulators create products that better match the sensory expectations of their target audiences.
Summing up.
To understand our somatosensory system is to understand ourselves. Our skin is the sensory organ through which we connect to and make sense of the outside world.
If, as cosmetic industry professionals, we wish to take our formulations further and create products that truly connect with their intended target audience it is imperative we consider the whole somatosensory system. That is, how we process touch both directly, through the skin, and indirectly through our other senses as well as our feelings and sense of self. Of course we have always done this to one level or another, but often subconsciously, occasionally from our, rather than our target audiences perspective (we like it so they should), or coincidentally and somewhat haphazardly through our almost exclusively ingredient-focused formulating lens.
Creating somatosensory products intentionally is the future and to help with that we should probably:
- Know our target audience as well as we possibly can, paying particular attention to their target-market specific window-of-tolerance.
- Do they typically light delicate or intense scents?
- Is their skin fragile or robust?
- Are they used to using skincare or not?
- Do they sit at the top or bottom of their window-of-tolerance as their ‘norm’
- Is my product designed to calm or stimulate them? If not, does it need to be/ is that a benefit?
- Formulate the whole product to this skin type.
- Choosing the right ingredients is part but not all of this. Ingredients alone don’t make a product.
- Pay close attention to the texture and how that may be perceived by the target audience.
- Marry up the texture and actives with the level and type and intensity of scent.
- Don’t throw sensory surprises that your client is not prepared for at them.
- Communicate clearly, appreciating that our window of tolerance is affected by our feelings of safety and how ‘in control’ and prepared we feel.
- Set customer expectations (use your label text, a product insert or your social media/ website to explain the what’s, where’s how’s and when)
- Consider options for dialling up and down the experience and communicate these to clients so they can choose and prepare themselves eg: fine, medium and harsh exfoliating scrub options; gentle, mid-strength and professional AHA serums. Light, medium and heavy cream textures.
- Continue the conversation.
- Carry out post-use surveys and focus groups.
- Continue to research your target market, stay open minded as to their sensory reality, commit to a mindset of continued growth and research
- Embrace the complexity and nuance that exists. Accept that you won’t be able to please everybody because we are all so complex and multi-dimensional.
My Somatosensory journey was not taken alone, many people were involved in helping me understand this aspect of my being.
In relation to what shaped this piece of work I would like to mention Somatic Social Worker Jo Mensinga who I ‘met’ and had some therapy sessions with through Instagram.
Next is Emma McAdam and her Youtube channel – Therapy in a nutshell. This channel helped me better understand the way the body stores trauma and what we can do to release that.
Both of these women reference the book ‘The Body Keeps The Score‘ by Bessel van der Kolk in their work and while I haven’t read it myself yet, I have read some of his papers on Post Traumatic Stress and have this on my reading list.
In terms of the technical/ biological aspect of our somatosensory system I’ve read a few different papers and looked at a number of websites. The main ones I took note of and notes from were:
- Hugo D. Critchley, Neil A. Harrison,
Visceral Influences on Brain and Behavior,
Neuron,
Volume 77, Issue 4,
2013,
Pages 624-638,
ISSN 0896-6273,
https://doi.org/10.1016/j.neuron.2013.02.008.
2) Somatosensory Tracts, Kahn Academy.
3) AUTHOR=Valenzuela-Moguillansky Camila, Reyes-Reyes Alejandro, Gaete María I.
Exteroceptive and Interoceptive Body-Self Awareness in Fibromyalgia Patients
Frontiers in Human Neuroscience
VOLUME 11
YEAR 2017
URL=https://www.frontiersin.org/article/10.3389/fnhum.2017.00117
DOI=10.3389/fnhum.2017.00117
ISSN=1662-5161
Academic Integrity & Cosmetic Science Communication
One of the surprising joys of being back at University (part time, very slow progress, yes I’m getting there but…) is the reminder that Academic Integrity is not only expected, measured and tested, it’s the law.
My uni – Charles Sturt, here in the beautiful central tablelands of New South Wales, Australia, has put together a five module course on the subject and we all have to complete this before being allowed to graduate. The course takes anything from 1.5 hours to a day to complete, depending on your own pace and level of engagement/ understanding. I had forgotten about this part of the course and only took the final tests on Monday and that’s what made me think of you all!
You can find a link to the CSU Academic Integrity Policy here.
The concept, practice and importance of Academic Integrity has never been lost on me. It’s something I deeply value and try my hardest to uphold while knowing and admitting I occasionally sip up over. One example of how I can slip up at times is in the way I reference my source materials on the blog. My referencing can present as a mash-up of styles and occasionally I might miss something that really should be referenced. Academically this is a no-no and could at best be seen as sloppy and unprofessional and at worst as rude and fraudulent. Neither sounds too good to me!
Academic Integrity covers a whole range of attitutes and behaviours, correct referencing being just one part. It also covers how we handle material that’s under copyrite or license agreements, how other people’s words are quoted (and how much of them you use), how, why and where you paraphrase, how you make notes, prioritise and scheduling tasks, seek assistance (and from whom), collaborate/ work in teams and so on. It’s about how you respectfully build on other peoples ideas when constructing your own arguments. It covers methods and tools for checking for plagiarism and authenticity. In a nutshell, it’s about carrying out and representing your work honestly and fairly, respecting the work of others and taking responsibility for acknowleging the influence they had on you and the role they played in helping you form your own ideas. Ultimately it is about trust – about trusting that other people will value and respect your work and that you can be trusted to do the same. Doesn’t that sound great? So how does that apply here?
Integrity, Inshmegrity…
On the one hand I want to rant on about how ‘anyone can write anything on the internet and some of it is just shit and wouldn’t know integrity if it bit them on the bum’
While on the other hand I’m thinking ‘there’s horses for courses Amanda. Don’t be so judgemental and anyway, you are anyone writing anything aren’t you? Get a grip woman’…
So as you can see, I’m quite confused.
I attempted to work through my confusion around the topic I’d just chosen to make a blog-post out of (and therefore should really already know the point I was trying to make) and that helped a bit but not a lot.
So in the end I decided to zoom out and see if that helped. It did…
Communication in Context
Academic Integrity is the communication standard that is required by Universities. It serves to uphold their position as leaders in academic thought and helps their people understand how to go about exporing, developing and communicating their ideas. There are rules and guidelines within Academic Integrity that can be monitored and measured and consequences for falling short. Your reward for compliance is to bask in your own awesomeness of doing what you should and having people not think you are a shabby, unreliable ass hole – sounds motivating enough don’t you think?
Outside of academia, the standards upon which our communication is measured differ. We all accept that we communicate to our bosses, doctors or religious leaders in a slightly different way to that we would use with our pre-school family members, our pets or the friends we just met for an evening out. Outside of pure ettiquete or familiarity there’s the other legal frameworks we operate in. Get pulled over by the police for travelling 10Km over the limit and it’s probably not a great idea to greet them with a ‘hello darling, I love what you’ve done to your hair’. Likewise, if you end up in court for any reason I don’t recommend you use flowery analogies or interpretive dance to explain your movements on that dark night back in October. There are rules and there are consequences.
Cosmetic Science Communication is broad and covers everything from the highly academic and scientific to the opinions and thoughts of the layperson so how do we apply Academic Integrity in this context? I did a bit of brain-storming around this and decided the best way to tackle this was to break it down into bite-sized chunks, starting with this, my blog.
The 6 W’s of (even though one of them is a H) Communicating via my blog.
Why do I communicate/ blog about cosmetic science stuff?
My primary motivator around blogging is to use it as a teaching tool – a platform for sharing the process of investigating intellectually stimulating topics in a way that might persuade and /or influence and/or inspire others into doing rather than just consuming. I write this for intellectual Stimulation, as part of my own thought and planning process. I also do it to show solidarity and support to anyone who feels anxious or scared about the chemistry that’s in their cosmetics. To demonstrate how one can think and go about investigating these things. Finally I do it because I find it somewhat fun and quite therapeutic.
Why do others communicate cosmetic science stuff? I think that’s up to them to tell you but I suspect that their authentic answers will be most interesting and useful when it comes to deciding how you engage with their content.
What am I communicating?
That is fairly obvious here, cosmetic science, issues surrounding the industry and products. Sometimes trends and movements or philosophies. Other times it’s ingredients and/or research.
How do I communicate?
I try to ground myself when I’m writing and by that I mean, to feel each step and make each step clear rather than make assumptions, skim over things or cover my tracks. It’s more about using that saying ‘walk in my footsteps’ as a metaphor rather than taking it in the literal sense (don’t look up to me, come with me).
Where do I communicate?
Well here we are talking about this blog but I do use other methods and they each have their own nuanced styles and rules I guess.
Who am I communicating to?
The people who read this blog are quite a diverse bunch (yes you are, aren’t you) but they typically all share a love of cosmetic science, a desire to go deeper and invest more time than most, and a curious, creative mindset. Some of my readers do get lost in some of the more technical aspects of the odd blog post but are still able to pick up and appreciate the gist.
What does exploring the W’s do for our discussion on acedemic integrity?
It makes it easier for me to identify the similarities and differences between my blog and the blogging community and a Science Paper and the world of Academia.
The Solution – Applied Integrity.
As is often the case, I find my point while writing rather than planning it ahead of time. It is as if the labour of doing uncovers the right path in a way that just planning can’t.
The value system that underpins Academic Integrity is deeply important to me both personally and professionally. I can’t teach the importance of ‘doing’ science, of prioritising creating rather than copying and exploring rather than following if I don’t myself use and demonstrate best practice. Well I could but it would be hypocritical.
Integrity is integrity and I share all the values my Academic Integrity unit laid out but I need to find a way to demonstrate it that’s appropriate for this, a more diverse and less ‘institutionalised’ audience. If people are intimidated or interrupted by my integrity steps they won’t keep reading/ watching.
How blooming obvious!
I feel like I should have known this all along and that the time spent working on this article has been wasted..
But wait, I HAVE been doing this all along, it’s just that I’ve been doing it sub-consciously up to this point!
Most of us know that feeling of being able to do something ourselves with ease, something that takes up almost no brain space at all, something that just seems to flow, to happen, to get done. Most of us also know how these simple tasks can start to feel way more complicated and involved once we try to teach others! Maybe you experienced this when bringing up or interacting with young children or helping others or yourself recover some every-day life skills after a brain trauma or illness. Well that’s what’s happened here. I’ve re-freshed my Academic Integrity skills, reflected on how they are demonstrated at Uni and realised how I try and make them work in a blog setting. So now what?
Consciousness Raising – Making Integrity Visible In My Blogging Practice
To do things consciously is to pay attention, to become accountable, to choose and to lead rather than follow and let be. I love all of that and am exited to have another opportunity to practive that!
I’m going to work on replacing my somewhat hap-hazard, covert way of demonstrating integrity with a more conscious, deliberate and overt approach. In short I’m going to make this aspect of my work more obvious and organised. I’m not doing it this way because I think Integrity should be shoved down everyones throats, nor do I think my readers are too dumb to notice if I don’t point it out this way. It’s more out of respect for the fact people read blogs for many reasons, not least to be entertained and inspired. That my readers come to the blog with different levels of experience around this topic and that some may not realise there is ‘best practice’ and etiquette around this type of work. I’m also doing this as I can’t very well moan about the internet being full of rubbish while not helping my readers learn to identify how to tell.
So that’s that. I’m off to come up with a little table-type box format thing that I can pop at the end of each article (outside of this one as I’ve not made it yet) that explains what actions I’ve taken, resources I’ve used and people I’ve been influenced by in the writing of my articles. I’m hoping that an added layer of consistency will help my readers appreciate the value of what we all share, create and explore together.
Thanks for reading.
This Working Life: Autism, ADHD, Complex PTSD and Getting Stuff Done.
I’ve struggled my whole life to understand why, in spite of my love of learning, doing and investigating, I get lost, stuck in a loop, overwhelmed or frozen all the time! It happens when I embark on a course, take on new projects at work, join a club or group and even when I sit down to ‘switch off’ with a movie or book. I just can’t seem to get the balance right, enjoy one thing at a time or participate on a piece-by-piece basis.
While this has been a constant struggle for me in my home and working life, I must take a moment to acknowledge just how far I’ve come and how much I’ve acheived both in spite of and because of this. This blog being a part of what I’d classify ‘my success’ in the broadest sense of the word.
In the business world, the ADHD tag often gets linked with behaviour that could be judged to be ‘immature’ or lacking discipline: impulsivity, flakiness, being a bit all-over-the-place or unpredictable. Being Autistic in business enjoys some slightly more positive stereotypes but they don’t quite compensate for the quiet ‘othering’ and weirdness that surrounds people like us. Our tendency to come across as socially awkward may make us seem eccentric, aloof, shy or disinterested while our amazing pattern seeking brains can see us as curiosities or have us framed as deep but narrow thinkers (the boffin or brain in the corner) but people who you wouldn’t want as part of your leadership team – who don’t often have anything ‘real world’ or practical to offer. The complex PTSD is a hard one – In business we should be able to ‘fake it until we make it’, show up as our strong, armoured self. But complex PTSD can rob us of our ability to know who we really are and make this game of ‘putting on our business self’ a real cognitive struggle – it may even trigger our trauma to do so.
The long and the short of it for me is I’m ready to get this part of me cleansed and treated. I’m sharing this video on here as the dysfunctionality I talk about helps explain what makes me both brilliant and chaotic, productive and stuck, creative and predictable.
I hope that in sharing this I can do my bit to normalise conversations around mental health in the workplace, help my readers understand how my brain works and where I might take this working life adventure in future!
Sustainable Palm: An ASCC panel discussion I missed out on being part of due to an I.T fail!
Last week I hit a bit of a low spot after missing out on this event.
I’ve had a lot stressing me out mentally for the last couple of years and only relatively recently felt on top of it enough to start participating in industry events, forums, talks etc.
I was accepted into the IFSCC (International Federation of Societies of Cosmetic Chemists) event to showcase my research which was amazing. Then had to deffer that as I couldn’t get the research finished due to COVID restrictions, a lack of funding, my exhausted brain and (as a consequence of overload) fragile mental health.
I was invited to talk at the Cosmetic Science conference in Thailand but had to pull out of that due to last minute confusion over dates and again me having nothing left in the tank to organise myself in time.
And now this.
This time I had prepared notes and was thoroughly briefed. Had practiced on the conference IT platform and had set up my profile and orientated myself. I was set up ready on the day and logged in to the site again and then boom. It all went wrong.
What went wrong is that I’d done my set up IT test at home on my desk top but was working away from home on talk day. I had read that the platform we were using wasn’t compatible with a number of browsers, tablets or phones but after having had no issues in the test round (or so I believed, I actually don’t know now I think about it) I forgot that detail again until the clock struck 3 and we were live. The system we were to use was not compatible with my Apple Mac browser! As it is a works computer I can’t change the browser settings without the help of the IT department who were off site at that time and (as it happens) struggling with their own issues due to the rumbling thunder taking out the internet where they were. Long story short I tried my Ipad (different browser) and got some but not enough joy. Tried my phone and had no luck at all, the other office computers and no- they all ran a browser that wasn’t supported. Before I knew it I missed 40 out of 60 minutes and had only managed a very brief appearance in the chat box and a few minutes of panel talk audio before I lost the whole thing.
These things don’t usually get to me but at the moment, probably because of all that’s been happening in life, it’s just a little soul destroying. I’m torn between becoming a permanent hermit or carrying on doing my best while risking letting other people down. I’m sure I’ll work through those feelings in therapy when the next appointment rolls around…
Once the dust had settled in my brain and idea came to me. While I don’t like the idea of hogging the attention or making this all about me, I did feel that I have some interesting things to add to this topic. It’s something I’ve been writing, thinking and participating in since my consultancy started in 2007 and even before that with my visits to Indonesia and Malaysia and since, with the work I’m doing on my own little farm out in the NSW central west. So I made this video.
I am sorry to have missed out on listening to the other participants who were (in their own words):
Maria Abadilla, Chairperson, Orangutan Alliance
Maria Abadilla is the Founder and Chairperson of the Orangutan Alliance an International Palm Oil Free Certification Program. In her role she leads the organisation’s strategic direction and works closely together with the Alliance’s Board and strategic partners.
Maria has over 20 years’ experience in product development and brand management. Prior to founding the Orangutan Alliance, Maria led her own consultancy working with Australia’s top 100 Franchise and FCMG businesses on strategy and product development as well as conservation projects.
Maria founded the Orangutan Alliance (palm oil free certification) out of passion for the preservation of the environment and its endangered species. Through the Alliance she aims encourage the investment and dialogue about alternatives to non-sustainable palm oil and advocate for transparent labelling and consumer choice on this issue.
With the palm oil free certification scheme the Orangutan Alliance looks to bridge the gap s between consumer demand, product development on the manufacturers side while creating a new revenue stream for conservation. Orangutan Alliance is part of the growing movement driven by consumer demand towards ethical and transparent labelling of products. Visit http://www.orangutanalliance.org for more information.
Moderator: Julian Jones, Managing Director, Ikonsulting Pty Ltd
Julian Jones, the founder of ikonique®, is passionate about the skincare industry in Australia. Julian has worked in the industry for over thirty years. He is widely known and respected both nationally and internationally for his knowledge and skills in developing and marketing the best anti-aging skincare. Julian has seen the skincare market make amazing progress over that time and is excited about the performance that is now possible when products are carefully and properly formulated and manufactured. Julian has applied his vast knowledge and experience to create ikonique®.
Louise Robertson, R & D Chemist, Ensign Laboratories Pty Ltd
Louise Robertson completed her Bachelors degree in science majoring in biochemistry in 2012 and has been working in the cosmetics manufacturing industry for almost 9 years now, she currently working for Ensign Laboratories as an innovation research and development chemist. Louise has a strong passion for the cosmetic industry, sustainability and innovation having experience formulating products from the ground up for many different regions, Louise is always keen to learn about how new and old technologies can be harnessed to create unique and captivating formulations. Louise has experience developing both palm free and sustainable palm personal care formulations to meet the clients expectations and the end consumers expectations.
Matthew Martens,Industry Sales Manager – Consumer Care Croda Australia
Matthew didn’t have a bio on the panel chat bit I got this from but he’s extremely knowledgeable, was the ASCC President for at least a couple of years and is an all-round decent chap who knows his stuff so I’m sure his contribution was as interesting as the others.
Anyway, this is me answering the questions as they were presented to me in an email prior to the event. I hope my belated contribution to the panel talk is worth some of your valuable time. Also on the YouTube video description I’ve linked to some of the articles I read while getting myself up to date with this topic.
One INCI List, Four Serums
I’m still thinking about that ‘turn and learn’ situation. The idea that an INCI list tells tells pretty much all you need to know about a product.
After writing my blog article the other day I felt there was some unfinished business on my part. I’m always telling people that science is a doing word so what better way to demonstrate my point of view than offer up a demonstration. So, sit back and prepare to be blown away by the magic of my one INCI list, four serums experiment! That is a joke by the way, I am always second-guessing myself around this sort of thing – did I come up with the best example I could? Will people see what I want them to see? Is someone going to miss the point entirely and write asking if the Blackberry extract can be swapped for Banana? Do I really need to finish the whole packet of biscuits? You know, that…
The Serums.
I put together what I could in the time I had with the ingredients available in my lab. I feel this does the job of demonstrating at least some of the limitations around the INCI-centric product evaluation strategy which is what I intended and focused on. This serum and its variants are designed for teaching purposes – to illustrate a point rather than to be something people might or might not like.
So here they are:
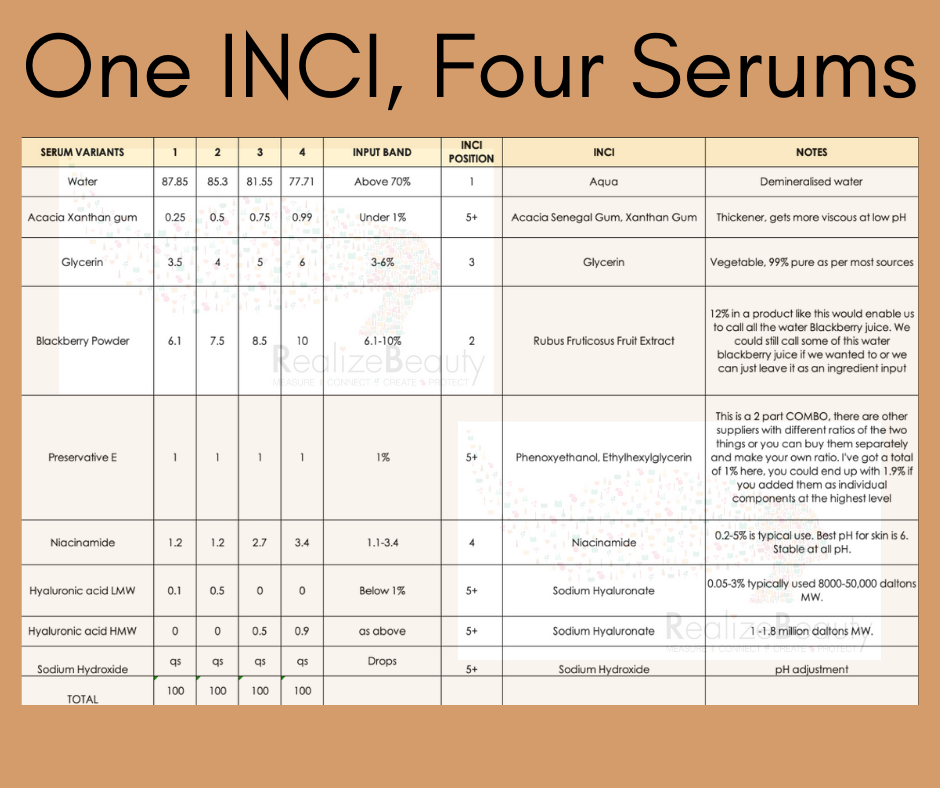
And here’s an INCI list to cover all four versions:
INCI: Aqua, Rubus Fruticosus Fruit Extract, Glycerin, Niacinamide, Sodium Hyaluronate, Acacia senegal gum, xanthan gum, Phenoxyethanol, Ethylhexylglycerin, Sodium Hydroxide.
What can the INCI list tell me?
That structurally these are a fairly simple, water-dominant set of formulas that contain a hefty amount of fruit extract followed by a couple of other well known dermatology-backed actives (glycerin, niacinamide and hyaluronic acid) in a preserved gel base. There is no fragrance or added colouring, the formulas are fairly natural (except for the preserative) and there aren’t too many ingredients.
Guessing what skin outcomes an INCI list like this might achieve.
I could assume the main function of a product with this INCI list was to re-hydrate and balance the skin with secondary functionality around environmental protection. The ingredients definitely support that with the glycerin, fruit extract and hyaluronic acid.
Then again I could assume this to be an anti-ageing formula with antioxidant protection highlighted and visible wrinkle reduction/ skin tone evening as second and third functionality. The INCI list supports that too!
Let’s not forget that it could also be a brightening formula! A formula optimised for gentle exfoliation and skin glow (fruit acids, Niacinamide).
Or it could be all-round face food product, a daily ‘respect your skin’ serum offering gentle,balanced all-round support.
But wait, it may be designed specifically to tackle pimples, marks and oily skin! The INCI list tells us the formula is oil free so surely it’ll be non-comedogenic. Also niacinamide is a really good option for pimple prone skin and break-out management.
And so we go on.
This, my friends, could be many things to many people depending on how these ingredients have been blended together and what the intentions behind it really were. This detail is often found outside the INCI list in the product description, directions for use, claims and product name.
Moving onto the actual formula versions.
Before we go too deep I wish to point out a few things about my INCI list (this is for anyone who likes reading things with a view to nit picking). There could be other ways of organising and presenting this including ways that would differentiate the variants as I’ll describe now.
Fruit juice powders, Aloe Powder and Coconut milk, water etc are often added to a formula and re-constituted. That is, re-hydrated with water to form their full-strength juice. That juice can then become an INCI ingredient in its own right thus reducing the level of ‘aqua’ (boring old water) or replacing it entirely. As far as I am aware it is not incorrect to list these ingredients either as their extract (refering to the powdered ingredient you added), their ‘juice’ (the power plus water to reconstitute it) or a combination of both. I opted for the former to help me make my INCI list point and also because not all powdered plant extracts have these options available so it’s not always relevant. Some fruit, herb or veg powders are not complete and so you can’t ever really end up with the equivalent of a whole fresh juice from them. I digress but yes there is this…
The second variation to INCI I’ll acknowledge here is in the ordering of the ingredients below 1%. They can go in any order so the one I’ve chosen is not the be-all-and-end-all. That said, it wouldn’t constitute a change in INCI anyway given they are all under the 1% mark.
If there’s anything else that I could have done I no longer care and am moving on (that’s not entirely true but I’ve got to get to the exciting stuff).
The making.
I opted to make these all the same way, cold process with a mixture of propellar and homogeniser mixing before checking and altering pH if necessary then packing off. I purposefully used the same method of manufacture as I didn’t want too many layers in this experiment but I acknowledge that chaging the manufacturing method, using heat, different mixing speeds and times etc would have an impact on the final product aesthetic for better or worse. Again these changes would not have been reflected in the INCI list and are another thing INCI can’t tell us.
The pH.
After just telling you I don’t want to over-complicate things I did do this. I split the batches into two and kept one at a pH of between 4.5-4.8 which is close to the natural pH this formula falls to due to the fruit acids in the Blackberry extract. I adjusted the other half up to 6.2-6.5 using sodium hydroxide (only a few tiny drops or 20% solution were needed per 100g sample) to better optimise the formula for Niacinamide activity. I did this because a) I just can’t help myself and b) you can’t tell the pH from the INCI list and there are plenty of examples where changing the pH can actually change everything with regards to formula stability, aesthetic and efficacy.
The Results:
Top from Left to Right: All pH adjusted to 6.2-6.5, versions 4, 3, 2 and 1.
Bottom from Left to Right: All pH 4.5-4.8, versions 4, 3, 2 and 1.
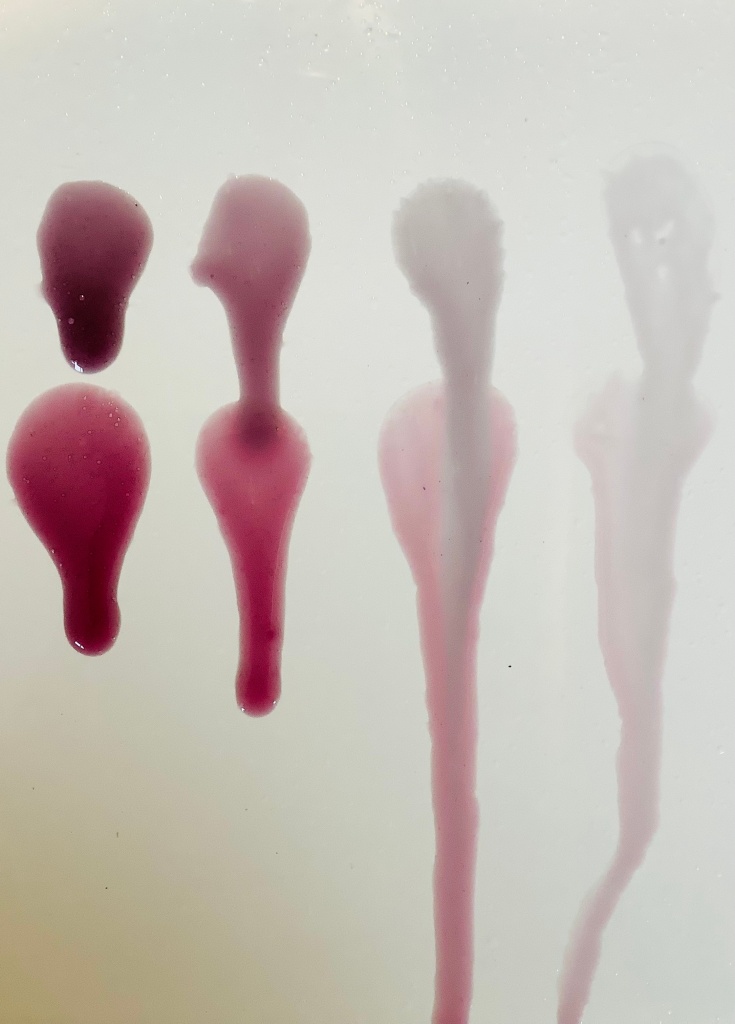
I haven’t yet had time to formally measure the viscosity but I am sure you can see that there is a clear difference between these versions just by looking at the pictures. This is unsurprising given the variation within the formulas but isn’t something the INCI list would be able to tell you.
You may also be able to see a colour difference. This is not uncommon when the colour of a product is created with natural materials such as fruit powders. The fruit contains a range of naturally occuring pigmented chemicals that respond to shifts in pH by changing colour and that’s what’s happening here – a shift from a pinky purple to a blue purple/ grey. This colour change may or may not signify a change in functional activity of the ingredient on the skin. We’d have to dig deeper into the chemistry of the extract to work that out.
Below are some other details I noted with regards to these same INCI, different outcome formula versions:
- I calculated the price per Kg for each version and there was 196% difference between highest and lowest – so the most expensive was nearly double the least all with the same INCI. Just in case you are interested, the recomended retail price based from the formula ingredient costs for these serums would be between $13.50 and $25.50 per 100ml of product which seems very good value to me – get me some! Oh wait, I already have them..
- The products at pH 6.2-6.5 would be best for activating the Niacinamide onto the skin but would definitely speed up the oxidation of any vitamin C present in the Blackberry extract. While the pH 4.5-4.8 samples would still lead to loss of vitamin C I would expect it to occur more slowly. If someone were to market this formula they would need to decide what to optimise the formula around noting the Blackberry extract is more than just a blob of vitamin C of course.
- Focusing on the Niacinamide we have a serum with nearly 3 times as much active niacinamide as another with no INCI list difference. It would depend on what I was claiming for this INCI as to whether that was significant or not.
- We can’t tell from an INCI list what molecular weight the Hyaluronic Acid is. In a cosmetic setting like this, the molecular weight of the Hyaluronic isn’t as critical as people might believe it to be with regards to its hydration and active delivery capacity. However, the Hyaluronic Acid molecular weight really does change the product aesthetic (viscosity, skin feel, speed to sink into the skin). Too much High Molecular weight HA, especially in a formula that also contains gum, can lead to it balling up on the skin and feeling yucky! Again, you can’t tell that from the INCI but you definitely would notice something off during application!
- Glycerin (plus the gum and HA actually) changes the osmotic pressure of the formula and the way it acts as a humectant. I wouldn’t expect the same performance, skin feel and osmolality from a product with 3.5% glycerin vs one with 6% glycerin and that could impact the formulations efficacy around skin hydration.
- Acacia/ Xanthan gum responds to pH getting thicker with decreasing pH to the point it can become quite glue-like. As the tackiness of a formula can be strongly influenced by surface-tension, the lower pH sample feels tackier (more sticky) than the sample at the higher pH. This wouldn’t be obvious from the INCi list and people might assume all the stickiness is from the fruit extract alone which would be incorrect!
- We can’t tell how stable any of the formula versions are from the INCI. A super trained eye might be able to identify potential problems in some cases and on a few occasions it is more obvious to someone with experience that a formula will perform below par, be unstable or otherwise vulnerable. Even in those situations it’s important to back up the hunches with testing as you actually never really know until you investiate hands on. An unstable product will lead to oxidation or mocribial contamination of the ingredients thus reducing its potential to do good on the skin and potentially making it irritating. So the INCI list can give us an idea around vulnerability but typically not enough to make a solid call. In this case I’d mainly be concerned about UV stability of the fruit extract, the vitamin C degradation impact and any thickening effect over time due to the formula osmolality.
- The INCI list tells us we have Blackberry fruit powder in the formula but it doesn’t say how the powder is produced, whether it is solvent extracted, hot or cold processed, how concentrated it is (how much you need to add to create a reconstituted juice, or even if it represents the whole dried fruit or just a part of it). This detail may or may not be critical in working out if the product contains the ingredients needed to deliver a particular outcome for the skin. As the specifics around outcome are usually listed outside the INCI list, those focusing only (or mainly) on INCI could make false assumptions about a product or just miss out on this valuable clarrification.
Summarising the state of things.
While I designed this experiment to point out the limits to the INCI-exclusive mindset I’ve been quite surprised at just how far those limits stretch even in a simple experiment like the one I’ve walked you through here.
To re-cap again, this two-part blog fest was inspired by the catch-phrase ‘turn and learn’ which in turn was something I came across on a YouTube beauty channel. The idea behind the saying being that it’s the INCI list that tells you most about a product’s worth and as such, is what we should focus our attention on.
The risk, when critiquing one tiny part of another persons work is that in removing it from its wider context you inflate or exagerate its importance and change the way it was meant to be received. That has not been my intention here, this is not a critique. What this blog post has been designed to explore is merely inspired by that catch phrase, delving into what might unfold IF we took the ‘turn-and-learn’ approach to extremes and took no notice of any other information about the product.
The last bit.
Most ingredients are active across a reasonably large range of input levels meaning they could appear at the start, middle or towards the end of an INCI list and still be functionally active and useful. Even ingredients with a relatively narrow active range can still fall into different slots in an INCI list depending on what else is in the formula. It’s really difficult to tell how relevant the ingredient placing is unless you are quite a skilled formulator and can de-construct and re-construct (reverse engineer) a formula with high accuracy. This is not as simple as you might think as we have ingredients like the Hyaluronic acid – same INCI, different molecular weight and aesthetics; ingredients like the preservative which is a blend rather than individual ingredient and ingredients such as the fruit powder which could change functionality depending on how it had been extracted and what part of the fruit it came from.
Factors outside of the INCI such as the product pH, viscosity, look, feel, smell even can affect how well a product works – you are less likely to use a product you don’t like than one you do and you might use more of something you love than something you don’t have a strong opinion over.
Then there’s the compliance side – has the formula been tested, does it make any specific claims or give you any clues as to what it was formulated to focus on, does it appear stable – how would you tell? Is it likely to have been made to good manufacturing practice standards, does the person who formulated it know what they are doing? So many questions.
Then theres the symphony side. I said in my original piece that cosmetic chemistry is like a symphony with many moving parts, something that’s alive, changeable and responsive to your touch. That type of detail is the X factor in many a formula and can never really be captured in words.
So that, my friends, is that. I hope I’ve made my point somewhat clearer and given you a few things to think about.
INCI is something, not everything and let’s work to keep it that way hey!
Amanda x
PS: I actually made this serum with the fruit powder because I wanted something colourful to look at. The other benefits are interesting too of course and the pH colour change is always a good thing to consider.
INCI Lists: Examining the ‘Turn-and-Learn’ mindset.
As much as I’m fascinated by cosmetic chemistry and the beauty industry I’m not really one for seeking out said content anywhere I can find it. I don’t tend to watch other creators YouTube chanels, read their blogs, hang around their schools or follow them on Instagram. I mostly read science papers and old-fashioned books (the ones with the pages that smell of libraries). If you are starting to think this may be because I look down on those ‘peasants’ that’s not true, well not entirely…
Sure, I do sometimes see and read things that leave me struggling not to bang my head against the wall and occasionnally I’m compelled to channel that energy into something constructive such as a blog article. On that, there have been times when my tone has been more ‘look here people, you are just wrong’ than it should have been but by far the most dominant thoughts and feelings that drive me are coming from a place of ‘I can see why you think that but I wish to draw your attention to this’…
Yes I’m definitely becoming more calm-blue-ocean as I age, but only time will tell if that’s because a) I’m all out of adrenaline and can no longer be bothered to fight or b) because I’ve ‘seen the light and it’s beautiful’.
With that in mind I want to talk to you about a promising young woman I’ve seen on YouTube called “Cassandra Bankston”.
I don’t know how I first came across Cassandra but I did and her words have been going around in my head like an ear worm for months now.
The early Cassandra tapes I saw were laid out as her providing ‘expert’ commentary on celebrity skincare regimen videos – a sort of video-in-video dissection service. As an aside, this style of content is something I enjoy and regularly watch Mama Doctor Jones do the same thing for ObGyno content. I put the word expert in commas in relation to Cassandra as while I don’t doubt she has suitable and even extensive qualifications I am not sure they run across all the topics she covers (Dermatology, Cosmetic Chemistry, Cosmetic R&D and Legislation, Beautician, Aesthetician etc).
It was interesting watching celebrities describe their skin type as they understood it (and some did seem very well educated in that regard) before explaining, often in great detail why they use and like the products featured. Most had clearly thought through their skin care routine in great detail – unsurprising given most make money from their looks – but this felt much deeper and more intimate than superficial and transactional.
In each video Cassandra put the celebrity on pause then shared her thoughts around what whas being said and done. It was all very interesting, a delayed call-and-response routine served up as a spicy dish of education, intuition, self-knowledge, pseudoscience, (potential) sponsorship or financial interest, opinion, best-guess, vulnerability and enjoyment. It all felt like harmless fun until I came away and couldn’t stop hearing these three words ‘turn and learn’. Noooooooooooooooo.
INCI Lists – The Turn and Learn Mindset.
Firstly yes, I do acknowledge the power of a good one-liner, saying or headline and secondly, I appreciate these things are often the tip of a much bigger iceberg and so we should always dig deeper before drawing any conclusions.
Cassandra uses the phrase ‘turn and learn’ not only to focus viewers attention to the back rather than front of the cosmetic product packaging (where the INCI list is) but to emphasise this as the most valuable information available to the general public. In the embedded video Cassandra explicitly advises people to completely ignore the front of the pack (which is something I didn’t know she had said when I first saw her videos). The idea is that once you have educated yourself on how to read and interpret the INCI list you can focus on that and in doing so avoid getting sucked in by sales consultants and marketing; avoid buying rubbish products; products that are not right for your skin and those that might irritate.
I use the ‘turn and learn’ strategy in both my personal and professional life. I have allergic reactions to a couple of ingredients and always check the INCI for them. I also check the INCI to get a feel for how the product has been put together (formulated) and I can gain some but not all insight into that from the ingredient list. On that last point, it’s worth noting that having formulated cosmetics for 23 years I have earned a level of insight that’s not normal.
What I find concerning.
The message that ABOVE ALL ELSE, it’s a products INCI list that tells us the most and uncovers its worth.
This is not just an over-simplification, it’s a complete dismissal of cosmetic science!
The cult of the ingredient.
These days, not a week goes by without an email that goes something like this:
‘Hi there, I’ve been doing some research and I want to formulate a cream for my clients. I know the ingredients I want in it (goes on to give me a list) and just need you to tell me how much of each I need for this to work’.
I NEVER used to get emails like this before social media and share-all edutainment became such a thing.
Emails like this show me a significant number of people who would otherwise be interested in cosmetic science think a ‘good’ cosmetic is just one that contains some well-known or cutting edge ‘good’ ingredients – the rest is just minor tick-the-box stuff you can cut-and-paste on. It’s like once you’ve identified and read all there is to read about the ingredients, you’ve done the hard yards and have hacked the skin science genome!
I don’t blame Cassandra for this, I doubt it’s something she’s even considered. She came way after the Campaign for Safe Cosmetics ‘Garbage in, garbage out’ campaign and they came after the whole ‘did you know, there’s SLS in the cleaning products they use in industrial car garages?’ faxes. There’s simply not a person alive who is powerful enough to change the collective mindset with one phrase or message. This, my friends, is an example of a slippery slope – an example of how cute little somethings, simple catch phrases and call-outs build on each other. They are like those single rogue hairs that fall out and land in your bathroom plug hole. Before you know it you’ve got a hair-berg blocking your plumbing and you had no idea how that could even possible!
Beyond INCI – Realizing the Intangible
It’s often easier to describe what something is not rather than what it is because you don’t have to understand a things essence to achieve a compelling ‘free from’ style analysis.
Cosmetic Science IS based on chemistry but being a great chemist doesn’t automatically guarantee you’ll make great formulations. Following best scientific practice is fundamental to cosmetic science success, but scientific precision alone doesn’t win hearts and minds. What about art? Cosmetic Science is artistic in the way it captures our imaginations and feeds our hopes and dreams! But again no, rely entirely on art and all you’ll get is a hot mess, broken dreams and a law suit…
When asked to describe what Cosmetic Science is in my classes I answer that it’s a relationship-building process, that it’s something we do and something we must apply!
Feeling this may still be a little too abstract I’ve sat here all day trying to come up with a cozy acronym that sums up what I feel cosmetic science is. I’ve persisted mainly because if I’m going to sit here whinging that the INCI list isn’t everything I’ve got to make visible this invisible ‘extra’ stuff – the special sauce. Doing this has really given me a numb bum so I do hope it’s useful. I ended up creating an acronym around my blog name ‘Realize Beauty’ which was definitely not my first choice and is at risk of coming across as corny but having brain stormed what cosmetic science meant to me this was the only remotely industry-linked word pairing I could muster and it sort of works.
In true me style, it’s not as concise as I’d like but it’s done:
The End Part
I once heard that art should make you feel something and it doesn’t matter if those feelings aren’t all good. I find myself in agreement with that and do see Cassandra’s work as art -she’s very good at what she does, I could never be as polished and professional sounding / looking as her and I really love that about her content and personality! There’s just something incongruous about this INCI situation which I don’t feel is at all intentional.
I’ve ended up being surprised by how much time I’ve invested into thinking about this ‘turn and learn’ situation. I’d not realised just how much each layer of a cosmetic product means to me and how much each layer contains its own opportunities and delivers value.
When I step back and look at a cosmetic product I see it as a symphony, something that’s complex, layered, alive and changeable rather than the one-dimensional and static. Something that starts in your imagination before guiding you towards a safe and helpful reality where the likelihood of you experiencing something good and worthwhile is heightened! Something that responds to the way it is moved, packaged, applied and then removed. Something that responds similarly but differently to you vs me because we are unique. A product-shaped relationship-building opportunity and one that is meaningful inspite of it being one that others might not fully appreciate or understand. If cosmetic science was just INCI list and fancy packaging there’s no way I’d be able to keep doing it.
So that’s it but before I go I want to make it crystal clear that I am not arguing against the usefulness of the INCI list and the insight that learning how to read and interpret them provides. Neither am I advising against watching Cassandra’s video’s or questioning her credentials. We all have blind spots (me included of course) and there’s no shame in that.
What I am trying to do is remind both myself and you out there that INCI lists are something not everything. A word of possibility awaits those who can see past the cult of the ingredient.
Amanda x
PS: Check out my next article where I share what I’ve done to make what I’m talking about visual. The article will be called ‘one INCI list, four serums’ and it’s ready now.
Amino Acids for pH Adjustment?
I question the wisdom of normalising the use of amino acids for pH adjustment...
Arginine is an amino acid and amino acids are famous for reacting together to build peptides and proteins. In a cosmetic sense, amino acids can be added into a formula individually to increase skin hydration or condition and strengthen the hair. In addition, they are frequently used as key actives in Mesotherapy cocktails where they are injected under the skin as anti-ageing and restorative agents.
Despite their family name, some amino acids act as bases (raising the pH) in a cosmetic formula. This fact has become increasingly interesting to formulators looking to raise the pH of their formulations in a ‘natural’ and biocompatible way, a way that avoids ‘fillers’ or ‘harsh ingredients’ or, in a way feels/ sounds more natural or organic. While the idea of utilising ‘basic’ amino acids for this purpose isn’t new and is, in fact indicated by some major cosmetic ingredient manufacturers, my concerns remain and have their origin not in the big picture but in the detail, the chemistry and that’s what I want to dive into here.
The problem of equivalence.
In terms of teaching, the promotion of Arginine as a drop-in alternative for Sodium Hydroxide for pH modification should stop. Chemically speaking, arginine has the capacity for introducing to the formula a level of chaos and vulnerability that sodium hydroxide simply doesn’t possess as we shall soon see.
The curious chemistry of Amino Acids.
Behaviour in solution (water)
Amino acids such as Arginine and Leucine behave mostly as bases in a cosmetic formula, increasing the pH of water when they are added. Arginine sends the pH to 12.5 while Lysine takes it to 10.5. This, and the fact both amino acids are quite stable in their structure across the typical cosmetic pH range of 4-8 are what make them suitable for considering as pH adjusters in the first place.
pKa value = A concentration-independent way of expressing the strength of an acid (or base). The lower the number, the stronger the acid, the higher the number, the more likely the ingredient will perform as a base. Ingredients can have one or more pKa values depending on their chemical structure and complexity. Ideally cosmetic pH adjusters would have either one pKa value only OR have more than one pKa but that they exist in a close pH band.
Amino Acids Vs The Rest.
Sodium Hydroxide is the gold standard for pH adjustment moving up (from acid to alkali).
When you increase a formula pH with Sodium hydroxide you are using a chemical that is very small and nimble (molecular weight 39.997g/mol). It neutralises to a salt and water, requiring very little addition to your formula (in most cases) to adjust a formula pH in the majority of cases. It is readily water soluble and has a predictable titration curve for the most part, although that will of course depend on what in your formula is making it acidic. As we will talk about pKa values in a moment, Sodium Hydroxide has a pKa of -1.8 and a pKb of 15.7 (correction, thanks Perry) meaning that in a cosmetic setting it will ALWAYS act as a base. The fact that it is a small molecule means it’s less likely (compared to Sodium Bicarbonate with a molecular weight of 84g/mol) to weigh down or salt-out (destroy) your formula. That the chemical reaction it undergoes in a neutralisation setting is simple (it literally makes water), highly predictable and efficient make worthy of a gold star!
When you use Amino Acids to adjust the pH of your formula you are using a molecule that is heavier, takes up more physical space and is more temperamental than Sodium Hydroxide. Arginine has a molecular weight of 174.2g/mol, Lysine 146.19g/mol and both have three pKa values plus an isoelectric point.
Isoelectric Point: A pH at which the Amino Acid (or other ingredient) carries no charge and so won’t act as a pH adjuster. In some cases we can put this to use as a pH buffering agent.
The three pKa values occur because the amino acids have three functional groups – the parts of the chemical that can participate in acid / base reactions. For comparison, sodium hydroxide and Triethanolamine have only one pKa value each (-1.8 and 7.74 respectively) while Sodium Bicarbonate has two (6.4 and 10.3).
On the amino acid, each group has its own discrete pH value where it becomes active (the pKa). The whole amino acid also has a pH point or range where adding more won’t budge the pH at all for a good amount of time (the Isoelectric point). This makes using them as pH adjusters quite complicated.
Having more than one pKa is not the end of the world. On the lowering-pH side we have Citric Acid, a commonly used adjuster with three pKa values (3.1, 4.7,6.4). Comparing that to other acids we might use to adjust pH down in a cosmetic setting there’s Lactic with one pKa (3.86) and Acetic (vinegar) also with one (4.76). Remember the lower the number the stronger the acid so in this case, for pH adjustment citric is strongest.
With regards to molecular size, Citric Acid is a good example of a common pH adjuster that’s also relatively large, sitting at 192.124g/mol. This is significantly heavier than the amino acids we discussed earlier, throwing doubt on the relevance of molecular weight as a measure of whether a chemical is fit-for-purpose as a pH adjuster. For reasons that will become more obvious as we go on, it’s fair to say that while molecular weight is worthy of consideration, it’s not the most important feature of the chemical.
Unlike Amino acids, Citric does not have an isoelectric point. It’s three pKa values are very close together and this is because its three functional groups share the same chemistry (-OOH or carboxyl groups). By comparison, Arginine and Lysine Amino Acids contain two types of Acid/ Base curious functional groups – the Ammonium group: NH4 > NH3+ and NH2+ plus the carboxyl group -OOH. Not having an isoelectric point makes Citric a much easier molecule to work with for pH adjustment. The absence of nitrogen containing functional groups is also a big winner as we will explore next!
The Chemical Reactivity of Amino Acids
Ammonium Ions and Nitrogen.
Amino acids contain ammonium functional groups. These are chemical bonds in which nitrogen is surrounded by hydrogen and bonded to carbon. In our amino acids, these nitrogen functional groups exist in what we call a primary position. Other options include having the nitrogen in a secondary or tertiary position. The naming relates to how many carbons the nitrogen is bonded to. Another nitrogen containing neutraliser that’s common to cosmetic science is Triethanolamine only this is a tertiary rather than primary amine.
Nitrogen chemistry is involved in one of the persistent ‘nasties’ issues in cosmetic science and that’s the presence or formation of Nitrosamines:
While amino acids, being primary amines are not the most likely nitrogen-containing molecules to form nitrosamines in a formula, their presence does create an opportunity for nitrogen-centric chemical reactions and not all of those are desirable. Again, I must point out that nitrogen is not ‘nasty’ or to be avoided – it’s abundant in our skin as I’ve already said. What I’m talking about here is the potential for a nitrogen-rich functional group to cause formula troubles and I’m particularly talking about this in the context of it only being in the formula to adjust the formula pH.
Here is a thorough assessment by the CIR review into nitrosamine formation.
Vitamin C goes brown faster with Amino Acids.
One common and simple reaction that can occur in your amino acid adjusted formula is the Maillard Reaction. This is where vitamin C reacts with the amino acid turning it brown and basically breaking it down.
Vitamin C is difficult to work with at the best of times as it is highly reactive and vulnerable to oxidation. Adding amino acids to your formula, especially those that aren’t strictly necessary introduces an added problem or layer of complexity in this regard. There are some interesting papers around vitamin C / amino acid interaction in the food industry:
Non-enzymatic browning in citrus juice
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4190239/
Non-enzymatic browning reaction in L-ascorbic acid/basic amino acid systems
https://www.scielo.br/j/cta/a/WZ63cgMzbDHR76hDqYQjzBq/?lang=en
Sugars break down with Amino Acids.
Many cosmetic formulations contain sugars and both glucose and fructose react with amino acids under normal storage conditions to form bi-products that go on to discolour the juice. While this is more an issue of aesthetic than safety it is still something most brands would prefer to avoid or at least minimise.
Your amino acid may form a Schiff base
Schiff bases happen when a nitrogen-containing compound forms a coordinate complex with a metal ion. I guess it’s similar to a chelation complex only more colourful and potentially less helpful.
Metal ions are not uncommon in natural formulations. They can come in on the back of your herbal extracts, your clays or your colour pigments. Should they find themselves attracted to and then bonded onto your pH adjusting amino acid your formula may change colour, become lumpy or granular or otherwise turn into a shit storm. Then again, this may not happen at all but as it’s possible, it should could be worth keeping in mind if your formula starts looking like a rainbow. You are not hallucinating!
Weird things happen when Arginine meets Citric Acid.
While I must confess to not yet being able to fully articulate what happens when Arginine and Citric acid get together, it is clear that in an aqueous environment they react in a way that’s non-linear and therefore somewhat harder to manage and/or fully predict in terms of pH response, formula aesthetics and stability. Citric is present in most fruit extracts and some herbal preparations meaning it might be in a formula even when you didn’t intend for it to be:
Unravelling the nature of citric acid:L-arginine:water mixtures: the bifunctional role of water
https://pubs.rsc.org/en/content/articlehtml/2021/cp/d0cp04992a
pH Adjusting with Amino Acids – A Problem of Extrapolation?
Amino Acids are OK or even preferred in some (specific) settings.
A great example of where the pH adjusting benefits of an amino-acid are preferable is Innolex’s Emulsence HC – a palm-free cationic conditioning wax that sends your formula pH to around 3 prior to neutralisation. I’ve used this ingredient in many formulations and can attest to the benefits of neutralising this particular ingredient with the suggested Amino acid vs Sodium Hydroxide or even bicarbonate of soda (which is pretty much always a dud choice). The amino acid doesn’t just work better, it is the only common option that works well, improving all aspects of the formula both in-use and in-pack.
Here’s a formula from Inolex showing how Emulsence HC is used to create a conditioning spray and how much Arginine is added to make the adjustment.
Lubrizol have also been known to mention amino acids in their technical documentation, listing them as one among many ‘neutraliser options’ for their carbomer family of polymers. While that appears on first glance like evidence for treating amino acids as just another pH adjuster that’s not quite the case. In this technical documentation Lubrizol are focused more on what can work for their polymer than what will work safely in your formula.
As we have seen above, Amino Acids can and do react with other commonly-found cosmetic ingredients so while it is correct to state there are amino acids that can adjust a formula pH upwards, extrapolating that to assume they work in all (or even most) cosmetic formulations likely problematic.
The Summary.
As much as we call cosmetic science ‘chemistry’, in many formulations we are actively trying to avoid chemistry happening! I mostly teach people to think of what we mainly do is closer to physics than chemistry – we help things exist physically together and share space without changing each other. We go to great lengths to avoid chemical reactions happening most of the time, adding preservatives, stabilisers, antioxidants, UV protectors and packaging appropriately. While pH adjustment is one of the chemical reactions we need, accept and invite, it is counter-productive to introduce an unnecessary layer of chaos at this point.
Do Amino Acids Even Delivery What We Want?
The fact amino acids are being widely considered for pH adjustment is a function of their perceived naturalness, gentleness and multifunctionality. While this is sometimes true, with some amino acids made via biofermentation, we can’t overlook the fact that many are synthetic in origin. Synthetic amino acids may or may not meet the criteria for addition in an organic or natural formula.
Price is another consideration for the cosmetic formulator albeit perhaps only a minor consideration. Amino acids are significantly more expensive than the alternatives (Sodium Hydroxide or Triethanolamine – although TEA has its own issues, some but not all of which are common to amino acids) and that’s without considering the extra R&D costs that could be incurred to thoroughly evaluate formula, packaging and micro stability after using this chemistry to adjust pH.
A third take-home point is the dose. The best pH adjusters are the ones that do their job very efficiently and at low addition rates. pH adjusters add complexity and ionic activity to the water phase and that, in turn can destabilise emulsions and de-solubilise solutions. For those reasons it is best practice to use the pH adjuster that does the job in the most invisible way and that is why we must consider aspects such as pKa, isoelectric point and molecular weight when weighing up our options.
Fourth is the reactivity – Amino acids are chaos when compared to the other typically used options. It is entirely possible that in adopting this pH adjusting strategy you are creating a formulations that has a lower shelf life than it otherwise might have. It may be more irritating, less aesthetically pleasing, less efficacious and less physically stable. Then again it may not. It’s all in the detail!
All in all, brands and formulators, teachers and workshop facilitators who are promoting amino acids as a more natural, safer or gentler alternative than Sodium Hydroxide for pH adjustment would be wise to think more deeply and adopt an applied rather than absolute mindset. I could be wrong, but I feel it likely amino acid pH adjustment could, in many cases, be a bum move.


