Yes Coco-Betaine and Cocamidopropyl Betaine are different chemicals.
Google stuff and a fair proportion of ‘research’ or information that you uncover will be either incorrect or completely rubbish. The above is just one example of how this incorrectness infiltrates our conscience and leads us astray. There are more ‘hits’ telling me that coco-betaine and cocamidopropyl betaine are the same than information confirming that they are indeed different.
I know that they are different because unlike the majority of Googlers I am a chemist. That isn’t a statement of superiority, it is merely an unemotional fact. There were very few people taking a chemistry major Degree when I did it and as far as I am aware it hasn’t gained much in popularity. Being a chemist allows me the special powers of understanding what a name means and I can see that the ‘amido’ bit in the latter chemical means this:
As pretty much all google searches for these two chemicals have been confused now I thought I’d put everything of interest on here so that you can see the difference.
In a nutshell the two have different chemical structures as I will explain below for those interested.
- The Coco-Betaine is a natural surfactant that can be used in organic formulations as long as it has been manufactured in an acceptable way, it contains no ‘synthetics’.
- Cocamidopropyl Betaine is always produced via a synthetic process although the coco part and the betaine part are natural.
- Coco-Betaine is more natural but it is also more irritating.
- Coco-Betaine is harder to track down than Cocamidopropyl Betaine.
- Both are surfactants.
- Both can be used in shampoos, body wash and other cleansing formulations.
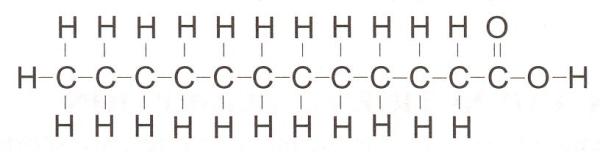
Cocamidopropyl Betaine Looks like this: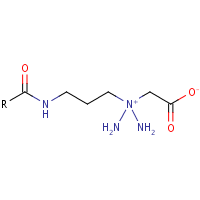
UPDATE 28.12.2018
Hello everyone and especially the chaps commenting below. I do seem to have copied and pasted a mistake with this structure which is not helpful for anyone and not exactly my intent when trying to stop mis-information spreading on the inter webs. In any case the structure above should be changed so there are two CH3 groups coming off the N+ and not two more nitrogens.
So, I’m sorry about that but while it was kind of annoying and incorrect the main point of the article remained correct in as much as Cocamidopropyl Betaine and Coco Betaine are two separate chemicals and that suppliers trying to sell the amidopropyl betaine off as the coco are incorrect and yes, that has been happening.
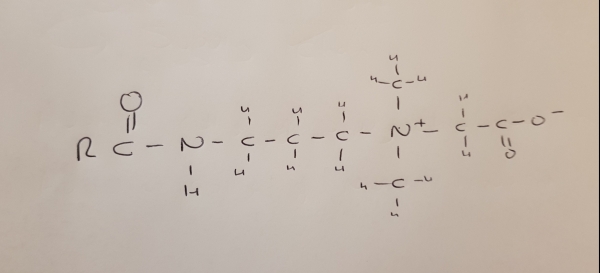
Coco Betaine Looks Like this:
Breaking it down further we have:
The Coco group: These are fatty acids that look like this:
The amido (we saw above)
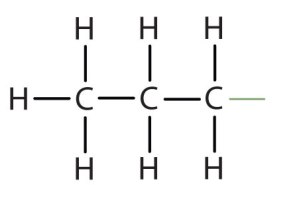
The propyl: Based on three carbons (Methyl =1 carbon, Ethyl = 2 (like ethanol), Propyl = 3). Below is a very simple representation of a propyl group.
The betaine bit: Betaine was first isolated from sugar beet – that’s why it’s called ‘betaine’!
So coco-betaine is a bit shorter (no propyl group) and is also missing the ‘amido’ functional group.
So what do they do in your formula?
Let’s let someone who sells both chemicals confirm that shall we!
And let’s look at how their responses compare when thickening a shampoo with salt:
It is hard to make good decisions about a products chemistry without some chemistry knowledge and it is doubly hard when people who are just ‘having a go’ post stuff that is wrong – usually without knowing.
I hope that this has helped clear up a little issue for you – it has for me.
So no, Coco-Betaine is not just shorthand for Cocamidopropyl Betaine.
Goodbye 🙂
Amanda x




hi amanda, which one can cleanse hair of silicones in order to not have to use a sulfate containing shampoo, i have heard that one of them or maybe both(I’m no chemist, I’m a nurse lol!) can cleanse silicone. thanks.
Ok so there are several things in this question to address:
Silicones: There are lots of different types, some that are more substantive than others. Most every-day type shampoos use silicones that can be washed out with a decent shampoo. SLS/ SLES/ Betaine systems are those types of shampoo as they are what we would call ‘harsh’ in Google language but ‘deep cleansing’ in cosmetic science language. Blends of these surfactants have good wetting and cleaning power and can disrupt the bonding of the silicone to the hair as the silicone is often cationic (positively charged) and the SLS/ SLES is negatively charged. The betaine is amphoteric and can flip between positive and negative charge.
Shampoo that cleanses without being too ‘harsh’: To achieve this you will need to maintain the chemistry above but possibly do it using less-efficient surfactants such as Sodium Cocoyl Glutamate or similar. These are less likely to irritate a very sensitive scalp.
Cocamidopropyl Betaine VS Coco Betain – both work the same but the Cocamido is a larger and milder molecule that is better tolerated for dry or problem skin. Coco Betaine is somewhat harsher. Coco is the natural version, cocamido is synthetic.
Also keep in mind that there are non-silicone 2-in-1 conditioner agents present in shampoos (Quats, polyquats) that will also build up in some hair. They can be cleansed using the same method here.
Much that is said about silicones in the hair is outdated now as technology has moved on significantly in the last twenty years.
Hi Amanda,
My 1.5 year old son has severe eczema and is allergic to cocamidoproply betaine (amongst several other chemicals). Should we also avoid products with coco betaine in them?
Hi Lyndsey,
That is a good question. I too suffer from eczema and was badly affected as a child so appreciate the importance of getting this right. Coco Betaine is more irritating a chemical than Cocamidopropyl Betaine so from that perspective it is probably to be avoided but that is not all. I asked a surfactant expert here (and representative of a Coco Betaine/ Cocamidopropyl Betaine) manufacturer) for their input and this was what he said:
‘The free SMCA (Sodium Monochloracetate) and free coco (amido) amine residues are the issue Amanda,Under the new GHS rules, both Cocamidopropyl Betaine and Coco Betaine are classified as category 1 for skin and eye irritation as well as aqua toxicity’
The advice I’ve got is to look at replacing Cocamidopropyl Betaine/ Coco Betaine with Cocamidopropyl Hydroxysultaine as that has no chloroacetate residue and is much milder. I’m going to start that process of converting customers over now and will be working with the surfactant manufacturers/ distributors to see if we can get some quantifiable results to help people with eczema make more informed choices. If you google the chemical name in the ‘shopping’ category you can find products that are already using this. The only thing I’d check before assuming the whole product is safe for you if it contains this is what other ingredients are present including fragrances and preservatives. Good luck! I’ll be posting more on this soon.
Some products have just ‘Betaine’ in the products listing. So does this mean ‘Cocamidopropyl Betaine’ or ‘coco Betaine’ ?
To complicate things further there is also an ingredient called ‘Betaine’. It is a humectant that also helps enhance skin penetration so it could actually be just that. If in doubt you could ask the brand owners but if you don’t want to do that you’d probably be best assuming they have labelled it right and it is the humectant rather than the surfactant.
Hi Amanda,
Thank you for this article.
Could you precise the reason why coco-betaine is more irritating than CAPB ? What is the source of this appreciation ? (chemical properties, biological tests… ?)
Thank you,
Elise
Because it is harsher. The Amide group was added to the structure to make it slightly less potent – all surfactants are skin irritants to some degree because they degrease the surface, in modifying the structure we can make them slightly less active and stripping. So coco betaine is more stripping as it is a harsher surfactant because of its un-modified structure.
I WOULKD LIKE TO TEST AND QUANTIFY THE CONTENTS OF BETAINE OR COCOBETAINE AND SURFACTANTS CONTENTS IN FORMULATED PREPARATIONS? ANY SUITABLE GUIDANCE PLEASE.
You will have to approach an analytical test lab to see what tests they can offer you for that. I know that testing to identify the presence of certain surfactants is possible but not sure via what lab or how much it would cost.
Hi Amanda,
Is CAPB easily wash off with a little water or even no water ? I mean, when i put it in floor cleansing liquid, do i have to rinse the floor with much water? Or do i have to clean the floor twice with fresh water?
The surfactant is designed to use diluted into a mixture and washed off with water. It is high foaming and can even be a foam booster so it might not be the right choice to use neat on a slippery surface like a floor. For that application we tend to use specially designed low-foaming non-ionics which hardly foam at all.
Can you give a suggestion for floor cleansing surfactant?
Wow phi loan! the conversation was about chemicals for the skin, not your floor!
Mop again if the floor is sticky right?
It’s OK V Short I would not feel morally outraged by Phi’s comments. I just answer the questions and comments in front of me without judgement and leave it at that. (well I try to). If Phi wants to wash the floor with CAPB or CAB I’m good with that. Maybe they can come and wash my floor too lol
Dear Amanda and V Short,
I’m so sorry if my question makes both of you feel unplease. It is actually that I have purchased a floor cleansing bottle. Its ingredient consists of CAPB as the only surfactant. And the sale man told that it is so safe and skin friendly when using surfactant for skin in household cleasing product. I try to figure out and I have already had your sincere answer. Thank you so much for that !
Hey not at all Phi! I was happy to answer, I took it as a legitimate question so please don’t feel bad.
ok I am fine. Thanks again for make me clear! That is so helpful to me.
hi amanda wondering what functional groups are present in cocamidopropyl betaine?
They are shown in the article 🙂
Hello! I am trying to look for a shampoo that has no SLS. And I found this company called “Trully Organics.” They used organic and all-natural ingredients, so I purchased their shampoo and love it. Although when I read the label, the shampoo includes these three ingredients (Sodium CocoSurfactant and Cocamidoprpoyl Betaine). I am not a chemist nor do I understand the interrelationship between different chemical and how they work together to produce a shampoo. But from what I have reserached that these 2 ingredients are synthetic surfactant and are harmful to our bodies. I have messaged them and they said, “All of the surfactants we use are all natural organically derived from coconuts. They’re are many different versions on the market which may be synthetic but not ours!” Is this true? Can both ingredients be produced naturally?
Sodium Cocosurfactant is not an INCI name so it is difficult to know exactly what that is. I’d go back to the company and ask them for the INCI name for that ingredient so you can make a more informed choice of whether you want that product or not. Cocamidopropyl Betaine is not 100% natural as described in this article. With regards to the comment about being derived from coconuts that’s a bit of a feel-good statement that can be true of many surfactants. The backbone (or bulk) of a surfactant can be made from any feedstock including coconut, palm or petroleum. Usually the feedstock will be whatever is cheapest and easiest to work with but some companies do try to sell on ‘green’ as in ‘sustainable’ platforms and might choose coconut over the others. But a surfactant is more than just the backbone, it also contains functional groups that make the ingredient surface-active. The feedstock that provides this functionality is what matters and often it is ethylene which is often from petroleum although these days more companies are producing ethylene from plant material. So it is complex. The bottom line is that yes you could get a surfactant that people on the internet claim is ‘nasty’ that is actually 100% renewable and ‘safe’. On the other hand you can get surfactants that the internet claims are good and that can be used in organics that are actually quite harsh on the skin and perform poorly on the hair. So it really depends on your situation and what you are trying to achieve.
I am wondering if it could also be less irritating in individuals due to it actually being natural & not a synthetic. As in the body itself responds better to natural meds, etc verses foreign/synthetic. Just a thought.
HI there Cee, I am yet to find evidence the body works that way – that natural is less irritating because it is natural. Chemistry exists within our bodies that is natural in one place and unnatural in another, helpful in the location it naturally resides but damaging when it moves – stomach acid is a good example as is endometriosis. It is a complicated thing to unpick but it seems less likely that naturalness is involved here – the less irritating version is the more synthetic too don’t forget. What is more likely to be the issue is the potential for the molecule to irritate the skin via one of a number of mechanisms and that is due to chemical structure, dose and regularity of exposure rather than where the molecules originated. After all, every chemical on earth is natural, it’s just that some haven’t been ‘alive’ as we know it for a long, long time.
On a technical level you are correct but on a practical level- not do much.
No one that makes cosmetics or shampoos or body washes wants to use a product that’s more irritating to the skin . So most would use cocamidopropyl betaine. Also most companies selling cocobetaine and or cocamidopropyl betaine have several versions available – look at all the versions just Stepan has . Most every day ingredients sales reps and home chemists use the two names as synonyms . My hunch is that cocobetaine was an early product that was modified later adding the amido and propyl to improve skin feel less irritation.
Well of course you are right Mark only not everyone has access to the information that you and I might have – I used to be the Product Manager responsible for Stepan (and way before that I looked after Akzo too). I wrote this article in response to me trying to locate Coco Betaine for a client who assured me that it was easy to get and better than Cocamidopropyl Betaine. I tracked it down to a small-lot supplier here only to find that they were selling Cocamidopropyl Betaine as Coco-Betaine thinking that Coco-Betaine was just a shorthand way of writing the latter. I wrote the blog post because I wanted a clear piece out there confirming that these are different chemicals and that while one is more natural (coco-betaine), it isn’t less irritation. Irritation score testing is something that only the big brands do. Smaller brands probably don’t even know how to go about that sort of thing and are actually very likely to put onto the market products that can irritate over continued use.
Thanks for bringing it up though as it is always good to have commentary on these articles.
Good accurate!!! Lubrizol’s CC O thickener works much better with the semi synthetic betaine.
That’s good information to have. Thanks!
I’ve been trying to make sense of the apparent confusion of these two products recently too, thanks for the article. Its not been easy tracking down molecular structures for them via the internet.
From what I could gather however, there appears to be some issues in the article above. The molecular structure in the article for cocamidopropyl betaine incorrectly shows 2 amine groups attached to the quaternary amine. These should be methyl groups instead. And the quaternary amine in the coco betaine structure should carry a positive charge.
Hi Kurt, Can you draw what you think the correct structure should be? I haven’t drawn the whole structure in one step, I’ve broken it down based on the parts that are used to make the final molecule so that people can identify what comes from where (in terms of sourcing). The Genamin structures are from the manufacturers of those ingredients so I’d assume they know what their chemicals look like but you never know. I just want to see what you are thinking so I can see if it makes better sense that what I’ve done.
Hi, Kurt is right. The molecular structure of cocamidopropyl betaine is not correct. The quaternary amine has two methyl groups instead of two amine groups. The molecular structure of the betaine (trimethylglycine) however is correct -> here the quaternary amine has three methyl groups.
The molecular structure of coco betaine also is not correctly displayed. The quaternary amine binds two methyl groups. That’s because they derived from betaine where the quaternary amine always have three methyl groups -> trimethylglycine.The displayed structure of Coco betaine here is more similar to sarcosinate-based surfactants.
Made some own structural formulas: http://kontis-online.eu/~julia/fotos/Betaine.png
Over a year ago since someone told you that your chemical structure was wrong and it’s still not been corrected. No wonder there is so much junk science and belief on the internet and this from a supposed “Professional Cosmetic Chemist”. Take a look at your structure for betaine and you will see two CH3 groups on the quaternary N. Now look you Cocamidopropyl betaine and you will see the two amine groups (NH2) on the quaternary N. Do you suppose you converted carbon atoms into nitrogen atoms when reacting??? if so the next noble prize will be yours.
As to your first comment “Google stuff and a fair proportion of ‘research’ or information that you uncover will be either incorrect or completely rubbish” yes, this page is a classic example.
As to your final comment “It is hard to make good decisions about a products chemistry without some chemistry knowledge and it is doubly hard when people who are just ‘having a go’ post stuff that is wrong – usually without knowing.”
Except you are “having a go” posting stuff that is wrong while claiming to be a “Professional Cosmetic Chemist” and don’t make any effort to correct errors when they are clearly pointed out to you.
Are you really a chemist? Do you understand chemistry? You write like you cared about people putting rubbish on the internet. Don’t you think you should make every effort to keep your website correct?
Hi Ian, thanks for the very harsh comments. Yes I really am a chemist but I don’t claim to be a genius or always right. I have made a note to look over this whole thing again tomorrow as today I’m out but if I have made a mistake I’ll correct it. With regards to your tone I can only assume that you have read my blog thinking that I think I am trying to paint myself as the new chemistry messiah and that’s simply not true. I am trying to get people to think and thinking (and telling me) I’m wrong if/ when I am is all part of that. What I don’t tend to do is re- write things as then none of the comments make sense and people can’t learn. What I’ll do here is make an update at the end of the article if I need to, to address your points.
Finally while I do appreciate the feedback I don’t appreciate your approach. It’s very rude.
I believe Julia has the structures drawn correctly in the link in her Feb 18, 2018 post. I believe the R- groups (“cocoyl residues”) should just be alkyl chains derived from coconut oil’s fatty acids, therefore most predominantly -C12H25 and -C14H29 (but other even-numbered lengths also between C8 and 18).
Hello and thank you for the information. It was VERY informative. One of my questions is i have been thinking of making a toothpaste for my business. Considering i know that all surfactants are somewhat of an irritant, i was thinking of replacing SLS for either coco-betaine or Cocamidopropyl Betaine that is commercially available. Are these surfactants a milder and safer alternative than SLS?
This article is so helpful, I’ve only found 2 other articles saying what your saying. That coco betaine and cocamidopropyl betaine are not the same!!!
Sadly many Many distributors market them as the same, with coco betaine being just an abbreviation instead of a completely different chemical makeup and products.
I am in search of coco betaine and am having such a hard time because of these other misinformed distributors. The only place I’ve found to buy it is from a company in India with the correct INCI name. My question is do you know of other places I could buy it from? Thank you.
In small quantities no, I can’t get it either. Clariant is still manufacturing but I’m not sure that any small distributors are buying. The biggest issue being that while it is more natural, it is also more irritating and less foamy.
La Roche-Posay’s Toleriane Caring Wash is said to use this yet is said not to be drying or irritating.
Irritating ingredients can be formulated into safe and non-irritating bases, it’s all a question of balance.
Thank you so much for this article…… several months ago I started experiencing an irritating rash on the back of my neck; and the tips of my ears were dry and flaky. I was also getting a sudden outbreak of psoriasis, therefore, all of my issues were lumped into the psoriasis category. I started thinking that my neck and ears were something else, and decided to check into a possible allergy to a new shampoo. My new shampoo listed both Coco-Betaine AND Cocamidopropl-Betaine in the ingredients. As you said, most hits indicated they were the same, so my question was, “why would this product list them both”. I am hoping I have hit the jack-pot in your article. I quit using that shampoo 5 days ago, and although I still have a rash, I feel that it is starting to clear up. (I certainly hope so, because the itching and burning have been miserable!) Thanks, again!
It can take a while to clear up inflamed skin so I’d go easy for a while. Also check the preservatives used. The scalp area can be more reactive than other parts of the body due to the active hair follicles being mini delivery ports, taking some chemicals in the hair products deeper into the skin than they otherwise would get. This is not usually a big issue but it can be if the product you are using contains ingredients you are reacting too. If the reaction was local it might be an irritation rather than an allergy. If it was a general reaction, that could be a sign that you have an allergy to something and that should be checked by a dermatologist as that can get worse over time. It is quite difficult finding out exactly what causes a problem when you have one but at least it looks like you are getting somewhere. I’m very sensitive on my scalp to preservatives and fragrances so I know how much of a pain it can be.
Wow – this is great, detailed information. I’m having a problem that maybe you can help with. I was recently diagnosed with a coconut diethanolamide allergy. The allergist gave me a list of the following ingredients to avoid:
• Coconut diethanolamide
• Alkanolamide of coconut oil fatty acids and diethanolamine
• Amides, N,N-bis(hydroxyethyl) coco
• CCRIS 4601
• Coco diethanolamides
• Coconut DEA
• Coconut fatty acid amide of diethanolamine
• Coconut fatty acid diethanolamine condensate
• Coconut oil acids, diethanolamine condensate
• Coconut oil amide, N,N-bis(2-hydroxyethyl)-
• Coconut oil fatty acid diethanolamide
• Coconut oil, diethanolamide
• Diethanolamine, coconut fatty acids condensate
• EINECS 271-657-0
• HSDB 4209
• N,N-Bis(2-hydroxyethyl)cocoamide
• N,N-Bis(2-hydroxyethyl)coconut fatty acid amide
• N,N-Bis(2-hydroxyethyl)coconut oil amide
• N,N-Bis(hydroxyethyl) coco amide
• NCI-C55312
• (Coco alkyl)diethanolamides
• Cocoyl diethanolamide
As I started reading labels, I found a lot of things with “coco” and “coca” in them that weren’t on the above list, so I looked at the hhs.gov website to see what these products were, and I discovered that in addition to all of the ingredients listed above, many of them have synonyms as well!
Now I’ve gone down a rabbit hole. If A=B, do I have to look up (and reverse lookup) every B to see if B=C?
All I want to know is if I have a coconut diethanolamide allergy, can I use products that have
• cocamidopropyl hydroxysultaine
• cocamide MIPA
• Disodium Cocoamphodiacetate
• Coco-Betaine
• Cocamidopropyl Betaine
• and other commonly listed “coco” and “coca” ingredients
or should i avoid anything that remotely resembles coconut oil?
Thank you in advance for any insight you can provide. It’s been decades since high school chemistry, so I’m stymied.
Great post👍, nice to find info from qualified people not just people googling stuff then passing it off as fact
Thanks 🙂
Thank you very much for the clarification of differences between coco-betaine and cocamidopropyl betaine! This is valuable knowledge and I greatly appreciate your time spent in posting this article. Keep posting because this type of information helps those of us researching for accurate ingredient information on the Internet.
Thanks for the feedback.
Hey,
Thank you a lot for this complex but understandable explanation. I unfortunately just discovered that I am allergic to Cocoamidopropyl-Betain and it is very helpful to know what similar sounding substances can be in a shampoo for me. Figuring this out on my own is not always easy, so thanks!
So does cocomidapropyl betaine fit more more into the cleansing / surfecant group, or can it also be considered an emulsifyer and foaming agent in a product?
It is pretty much just used as a surfactant for cleaning.
Thank you Amanda. I suspect that where the ingredient appears in the ingredient list somewhat determines the strength, i.e, if way far down in the list, Cocomidapropyl Betaine might be more of an emulsifier/foaming agent if it was way far down the list, let’s say in a hair mousse, as opposed to a cleansing condtioner?
A surfactant can boost foam, sometimes help with emulsification and act as a solubiliser. In a hair mousse it would boost foam. That isn’t so much a function of where it sits on the INCI list but more of what the formula needs and formulator selected it for.
Hi
You mentioned above “The Coco-Betaine is a natural surfactant that can be used in organic formulations as long as it has been manufactured in an acceptable way, it contains no ‘synthetics’”
What do you mean by the “acceptable way” and what synthetic could be in it?
Thanks
I mean it hasn’t been made using synthetic additives
Thank you,well explained.
Hi, I formulate a leave on conditioner for may pet. All of the ingredients i used are natural except CAPB, Can I no longer claim this product as “All Natural”?
The word ‘natural’ is quite ambiguous in the Cosmetic world. I think you would have to think about what you were trying to achieve, what your marketing is really saying to clients and what the law (if there is one in your country/ market) states. Often people apply for third party certification such as Cosmos natural or Ecocert. If you can use ingredients like this in those standards and still be natural that should be enough.
I would say that all surfactants are synthetic – they don’t appear in nature 🙂
The only “natural” ones would be yucca extract or other plant extracts with saponins.
If you look through the approved ingredients list for COSMOS (http://www.cosmos-standard-rm.org/verifmp.php) and search for it, you will find plenty of suppliers for Cocamidopropyl Betaine which are actually approved.
Which is better for soft skin, coco betain or CAPB?
The article covers the pro’s and con’s of each. Cocamidopropyl Betaine is milder than Coco Betaine so maybe use that as your starting point.
Hi Amanda,
I’m so happy I found your arfical, it is so confusing on the web…
I want to make shampoo for my family…
Which one is healthier?
The natural that irritates -coco betaine or the syntatic with the coco amido betaine?
Thanks a lot,
Michal
Neither is healthy, its about formulating to get a product that performs well so you need less to do a good job.
Some R&D say Coco-betaine is low ordur than CAPB. Loreal shampoo system uses coco-betaine but most of shampoo uses CAPB as thicker and form booster.
The odour would most likely be due either to any contaminants in the mix (there are always some, not all would be problematic but higher quality surfactants usually have lower colour and smell), or because of a preservative they use in the ingredient to keep it shelf stable or because of the concentration. All chemicals have a smell of something even if it is quite mild so it would be important to dig deeper into this to see what ‘smell’ they are talking about and whether it is indeed relevant.
Thanks! This was very helpful. I’m allergic to the synthetic longer version. Glad to know coco Betaine is different.
Hi there,
Thanks for that. Don’t forget the Coco Betaine is substantially more irritating so while you may have an allergic reaction to the CAPB, you might get an irritation reaction to the betaine so do check for that and be careful.
wonderful ..thank you for the clarification, unfortunately the suppliers here tend to roof it under the same thing. I was hoping the CAS number would be a good way of differentiating them. Is that a reliable way?
Hi there, yes the CAS number should be a reliable way to differentiate as each chemical should be sold with its unique CAS identifier. However, sometimes this fool proof system is stuffed up. Cocoa Betaine CAS 68424-94-2 Cocamidopropyl Betaine CAS 61789-40-0
From what I understand, amidoamine is a byproduct of cocamidopropyl betaine. I found out that I am allergic to amidoamine and could be allergic to products with this. Does this mean that amidoamine is not a byproduct of coco betaine and that it would be safer to use products that list this? I’m sure you can’t really give medical advice, but I’m just trying to figure out what I can try (or rule out for sure) because having this allergy makes it nearly impossible to find products I can use. Particularly shampoo. Thanks for your help!
I mean safer as far as allergies go. I know you said coco betaine is more irritating, but hopefully I’m not allergic to it. Just wanted to clarify 🙂
I would have no idea. Allergies are an immune response and those are very personal. You would need to get tested.
In reference to silicones. Please do keep in mind that silicon is an element just like carbon or chlorine. It binds with other atoms to make molecules. Some very good and some can be poisons. Each molecule should be evaluated on its own merits. Please do not vilify everything that has silicon in it. In fact silicon has special properties with amazing possibilities for cosmetics. It makes up 78% of the earth’s crust and all the computer chips.
Example I have seen on the internet, chlorine is found in both bleach and Sucralose. So Sucralose must be bad. But chlorine is also found in table salt (sodium chloride) and you cannot live without it. Good explanation here about Sucralose: https://www.acsh.org/news/2016/06/16/chemists-were-wrong-about-splenda
What the is the percentage of using coco betaine in dishwashing liquid
It works good with labs ,sles, or dea?
I came across your article while researching the differences between cocamidopropyl betaine and coco betaine because my family has suddenly developed an allergy to a liquid soap that we have used for years. We also noticed a sensitivity to a dish soap that we have used without issue in the past. We found an alternative dish soap that we did not react to, so we compared the ingredients. What we found is the synthetic version causes a reaction, and the natural version does not. Given the fact that the natural version is said to be a greater irritant, I found this interesting. We can use soaps with coco betaine and have no adverse reaction, but if we use a soap with cocoamidopropyl betaine, our hands itch, become red and irritated, and even start to crack and bleed with extended use. Just sharing our experiences in hopes that they may help someone else. Have a good day. Thanks for your writings. Cheers! Michele
Hi Michele,
Thanks so much for that.
What you are telling me does sound interesting and worthy of a full investigation (if you are up for it).
Before I carry on I would say that just because on paper one chemical is more irritating than another, it doesn’t mean that you will experience that response in practice. Also there is the chemical and the contaminants. The CAPB can contain contaminants from the manufacturing process. This is especially true when the ingredient is of low quality but is unlikely where the ingredient is of cosmetic grade or higher.
Ok with that in mind it would still be good to check your theory for real, that is that the CAPB is more irritating to you than the CB.
Testing bought products is not ideal as they are unlikely to contain only one variable – the CB or CAPB. Also, it is possible that what is listed as CB on the label is actually CAPB as I’ve seen that mix up before.
To set up a controlled test you would need to purchase a good quality (cosmetic grade) batch of CAPB and CB plus some demin water and a thickener gum (probably xanthan would do as that’s easy to get).
You could make up a 1% thickened water gel and split it into 2 batches, to one add 5% of the CAPB and label it, to the other add 5% of CB.
When I say 5% make sure that’s 5% as actives rather than 5% as supplied as surfactants are often diluted – you really want the two samples to be very accurate.
You could then test one on one side of your body and the other on the other.
This isn’t perfect and it certainly isn’t a blind test (you will know what you are applying so may have a bias towards one or the other outcome) but it is something.
If you do this testing on the day you make the batches you won’t even need to preserve the products. Preservatives are a big source of irritation and so are fragrances so avoiding them in your testing is essential.
I’ll leave that to you anyway as you may find doing that interesting.
As a cosmetic chemist I want to assure everyone that company’s that make cosmetics like shampoos and shower gels always insist on using cosmetic grade chemicals. These chemicals are treated to remove objectionable components. I screen the chemicals that are purchased by my company. They are first of all the best quality. We have partnered with a manufacturer that has high standards and a solid reputation for high quality.
Hi Walter,
Thanks for your perspective but remember that I am speaking and writing from the perspective of a cosmetic chemist too in a manufacturing environment and space that deals with lots of small brand owners (and bigger ones but they have less of a problem with this). It can sometimes seem so easy for us but it isn’t for everyone, especially when not all ingredient suppliers have the education and systems we have been educated to use and apply.
Yes Maverick Packaging Inc., as a contract manufacturer and packager, deals with both small and big brand owners as well. Some are just startups. Some are big and supply Walt Disney, hotels both high end and cheap and Airlines. However I think anyone can go online and look up CAS numbers using Google search. And also anyone can and should demand USP, NF or cosmetic grade from their suppliers. My intent was to arm people with this knowledge.
You are doing a lot of selling of your company. That’s what is coming across you me. This blog is not designed for the promotion of others, just for education so please try and comment with information rather than self promotion.
Was not intended. Wanted to address your original criticism that we only dealt with large clients. But don’t worry I will unsubscribe. You seem to always find only negatives.
Ok, goodbye 👍
One other point I would make is that when trying to decipher different chemicals it is best to use the CAS numbers instead of INCI names or any other kind of naming system. CAS numbers are much more specific. They can help you decipher the difference or similarity of the Coco Betaine VS the Cocamidopropyl Betaine. The same INCI name can have multiple CAS numbers. I did Immunodiagnostic R&D for 23 years. I did not even know what an INCI name was until I started doing cosmetic chemistry. This is because CAS numbers are better at identifying a chemical. Also the grade is important. In Immunodiagnostics we exclusively used ACS grade. But the cost would be prohibitive for cosmetics. Cosmetic chemicals are usually USP or NF grade or simply called cosmetic grade.
That’s good in theory and perfect for larger companies buying from distributors who have good document control but not all the smaller ingredient suppliers operate that way. I’ve seen a few occasions where chemicals are mis-labelled and this was one example.
just came across this blog, very helpful. i tried a shampoo called ControlGX (if you look at reviews for it anywhere you see people talk about their hair falling out and thinning over and over) this happened to me the moment i used it in the shower and i have very thick hair, i’ve never experienced anything like it, my hands were covered in hair. a year later i just bought a new shampoo and had a very similar reaction. checking the labels i can only see 3 exact identical ingredients.
decyl glucoside
phenoxyethanol
cocoamidopropyl betaine
could you give me any insight into these 3? thanks for taking the time to post this stuff.
Thank God! Finally a chemist to the rescue to this issue (fellow chemist here), came across your publication, there is so much “information” in google and somehow everyone becomes a chemist and formulator overnight, it takes years of study, degrees and constant update for new materials to truly understand chemicals and its effect on consumer products. I’ve seen people formulating “natural” products that become a hazard to the public, such as facial care, sun protection etc. Please everyone, make sure to consult with a real chemist to achieve good and safe products for your buyers, it is science and it requires care and knowledge to do them, don’t guide yourself with only google searches, many fellow chemists out there that will help you out like Amanda here. Thanks for sharing this Amanda!
“USP grade” is not a grade. USP is a grade system. USP includes “pharmaceutical grade,” “food grade,” and “lab grade,” among others.
^ Are those assertions true? Some clarity on Grade claims on products would be helpful to shop less-poorly informed.
that was not meant to be a reply
Does saponification of coconut oil produce the much dreaded Sulfates in soap or shampoo products?
I like my hair but the old people have the gene for MPD. Once I get to that age I would like to have not endangered my hair with follicle damaging surfactants.
The dreaded sulfates can be created from any saponified oil so yes maybe but not always. I am not sure what you have been reading but if you have a gene for something I feel that your choice of shampoo (or otherwise) would only ever be able to do so much.
Hi, I was researching a product and looking at the ingredients and same across this article, it’s great thank you for writing it. I wanted to know ‘if’ there was a reaction to a product which ingredient out of the formula would cause that. I know that some ingredients may not make sense to me in a formula, but might have a function from a formula and chemist point of view. What do you think of this formula? (It’s La Roche Posay Caring Wash)
AQUA / WATER • GLYCERIN • PENTAERYTHRITYL TETRAETHYLHEXANOATE • PROPYLENE GLYCOL • AMMONIUM POLYACRYLOYLDIMETHYL TAURATE • POLYSORBATE 60 • CERAMIDE NP • NIACINAMIDE • SODIUM CHLORIDE • COCO-BETAINE • DISODIUM EDTA • CAPRYLYL GLYCOL • PANTHENOL • T-BUTYL ALCOHOL • TOCOPHEROL
As you can see it contains coco-betaine 😉
Thanks!!
Ok first, it is not that simple. While there are ingredients that are more likely to cause a reaction in prone people (colour and fragrance for two), that doesn’t mean that everyone who experiences a ‘reaction’ will be experiencing it because of those things. I think it’s important to think about what reacting to a product means as it can be many things. If I assume you mean negatively responding to the product, that could have aesthetic, microbial or chemical trigger(s). So people can react badly to a product that irritates them because of how it feels or smells. I’ve had people reject samples because they perceive them to be harsh because they don’t like the smell for example. So delve into that further first. Then if this particular product was causing a problem for a person and it was definitely an ingredient to blame (so not pH, use instructions or any other factor), then the most likely chemicals to react to would be propylene glycol, coco betaine, caprylyl glycol, panthenol or tocopherol. Of these, the first three are more likely than the latter two to cause issues but all have triggered irritation in the past. So it can be tricky. Finally keep in mind there is a difference between a topical and systemic reaction. An allergic reaction may send a person into a full-body response whereas brushing against or using a product that irritates should only cause a reaction where it touches. Hope that helps
where is the english for the lay person to know what is “natural” vs not? this is why it is so hard for those of us with severe contact demititis to find safe products to use
What’s being natural got to do with it though? I also have severe dermatitis.
Can i add Cocamidopropyl Betaine to Methyl Ester Sulphonate + water + salt solution to make laundry detergent? Scared it causes undesirable reaction.
What have you heard to make you think this will cause a bad reaction? All things being equal I can’t see this being a problem but you should always test your ideas/ formulations out before you scale-up and especially before commercialising them.
what a useful article, i always wanted to understand the difference from chemist point of view, although i do have a query, how do exactly coco betaine are manufactured from coconut oil?
Thank you,
The coco part refers to the coconut fatty acids – so rather than the coconut oil be saponified and fractionally distilled into its individual fatty acid chain lengths, coco means either all or the majority (or main) fatty acids in coconut are used to make this surfactant. We would classify this as a ‘broad cut’ vs a ‘narrow cut’ or ‘specific’ chemistry. Surfactants made this way are often cheaper to make as there’s one less step to do (no need to specifically sort the fractions of fat). In terms of functionality, broad-cut surfactants are slightly more variable in their performance so less precise but that isn’t always an issue. The Betaine part is amino acid derived with the original ‘betaine’ found in sugar beet. In nature Betains are used as osmolites, water regulators. In this case, it’s the water-loving head of the surfactant that bonds onto the fatty acid tail to create a soap.