When making sunscreens particle size matters.
It’s that time of year for us Aussies where the budget is stretched, the sun is hot and commercial sunscreens are no longer cutting it – too expensive, itchy, heavy, fragrance issues, stingy, full of ‘nasties’ and the list goes on.
I have had numerous conversations with people wanting to make their own and usually this involves an attempt to blend either Titanium Dioxide or Zinc Oxide into a base.
My advice is always the same. Don’t try it, it doesn’t work……..
There are several reasons why making your own sunscreen is a bad idea. I have covered many of them before in this article ‘the trouble with making your own sunscreens‘ but I now have something else to add, and it is about particle size.
One of the things that home-made sunscreen people are keen to avoid are nano particles.
Nanoparticles sound man-made, potentially dangerous, different, weird even.
The organic standards that I am aware of don’t allow them – well, not man-made ones anyway.
The scientific jury is still out on some of the finer details of the long-term health and environmental implications of using nano particles in cosmetics.
Which begs the question, why do we use them?
Well first of all let’s get a little more clarity on the term nano particles. In terms of the cosmetics industry we have a general understanding that a nano particles is something that is sized between 1-100nm.
You could fit around 88,000 1 nm large things across the width of a human hair.
Or 880 100nm large things in the same space.
But it isn’t just about size. There are some other bits of data that matter when it comes to defining nano particles. Here is the EU official definition:
‘an insoluble or biopersistant and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 to 100 nm’.
The part about the particles having to be insoluble is very important. Insoluble in what? This is an applied measure in as much as solubility must be relevant to the ingredients application and fate – water, oils and other substances that it will come into contact with along its journey intended or otherwise.
The part about its biopersistance is also highly relevant – will the nano particles remain in the body, accumulate, become toxic etc? Will they make their way up through the food chain? Will they mutate and turn us all into zombies?
Intentionally manufactured is also worth noting as nano particles do exist in nature. This isn’t all science fictioning!
Anyway, as you can sort of see from the above, defining a nano particle isn’t actually as easy as shouting ‘hey, there you are!’ and noting it in your nano spotting journal. It is actually quite tricky.
So with that in mind people like the guys at Australian Certified Organic have decided to stay well away and have gone with a minimum 300nm size restriction on their permitted ingredients. Now while this means that products certified by the ACO will contain no nano particles it doesn’t necessarily mean they will be safe, especially when it comes to sunscreens.
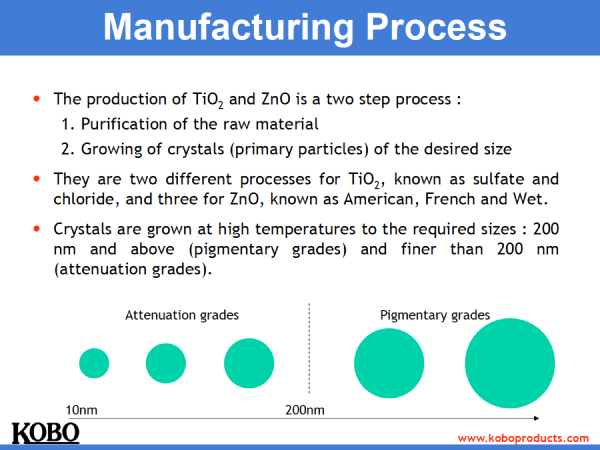
The Zinc Oxide and Titanium Dioxide that are really good at protecting us from the sun have very particular chemical and physical properties that make them so efficient and are referred to as ‘attenuation grade’ materials.
In this situation attenuation is a word used to describe how the particles reduce the intensity of the sunlight that falls upon them. The higher the rating the better. Wikipedia actually has a good definition for this word which I have copied here:
“In physics, attenuation (in some contexts also called extinction) is the gradual loss in intensity of any kind of flux through a medium. For instance, sunlight is attenuated by dark glasses, X-rays are attenuated by lead, and light and sound are attenuated by water”
As an aside I am always amazed by how much more time I spend trying to get my head around the Physics rather than the chemistry of cosmetics………..
Now a funny thing happens with Zinc Oxide and Titanium Dioxide as their particle sizes get bigger. They become less effective at attenuating sunlight. In fact they become quite rubbish.
Here is a graph put together by ingredient manufacturer KOBO to explain how the particles are made and to show roughly how the size of pigment zinc or titanium looks vs sunscreen grades:
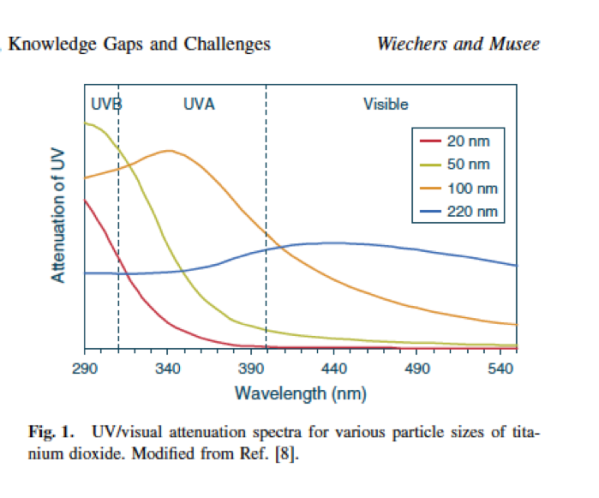
and here is a graph to show how the particle size influences attenuation of sunlight. I picked this graph up from an article put together by Johann Wiechers (deceased) and Ndeke Musee. The rest of the article is really worth a look too.
Basically on the above graph, the higher up the attenuation scale the filter the better. As you can see 220nm Titanium Dioxide has a flat line towards the bottom third of the graph. This means it is not good as a sunscreen. EP/ USP grade Titanium Dioxide and Zinc Oxide have particle sizes around 300nm.
So what does this mean for those of you wanting to make your own sunscreens?
In addition to what I have said before about this being hard to do and expensive to test you should also consider this:
- When it comes to UV protection, the smaller particle sizes are the ones that work to reflect UV, larger particles that sit in the pigment grade or USP grade are way less effective and may even be worse than having nothing on at all.
- Both Zinc Oxide and Titanium Dioxide are photoreactive. Intentionally manufactured sunscreen grades of inorganics are coated to minimise this photocatalytic activity, USP/ EP grades are not coated. This again may make your product worse than useless. You may end up walking around with a UV reactive catalyst on your face. Now that is scary!
You should also consider this other point which I haven’t delved into before but is worth a mention.
- Finally there are two common particulate shapes of titanium dioxide – rutile and anatase. Again sunscreen manufacturers know that the Rutile form is safest and usually stick to this source for their sun filters. This isn’t the case for pigment grades which may not have been destined for sun or even cosmetic use when mined or manufactured.
And that is why I say no. I’m not just being mean.
Take care.
Amanda x


So, commercially produced/sold cosmetic products that state SPF 16 through SPF 30 (powdered foundation/powdered bronzer/blush) that contain TiO2 & ZnO May not actually offer SPF protection? They’re non Nano and offer a pigment/cosmetic effect.
Hi Kathy,
If the lab method used to test the product is out of step with how the public actually use a product then yes, that could be the case. So the product may have a tested SPF that is accurate based on lab method but is never achieved in practice. I would imagine that this is something that scientists are looking into but as there is a need for a standardised and reproducible test method there will always be some people who apply differently and therefore achieve a different result. On the plus side though it is OK for brands to have their products tested to an SPF 50 say then only sell them to SPF 30 after doing a bit of work looking at how they may commonly be used. So it isn’t all doom and gloom and companies with SPF products generally do care about this sort of thing.